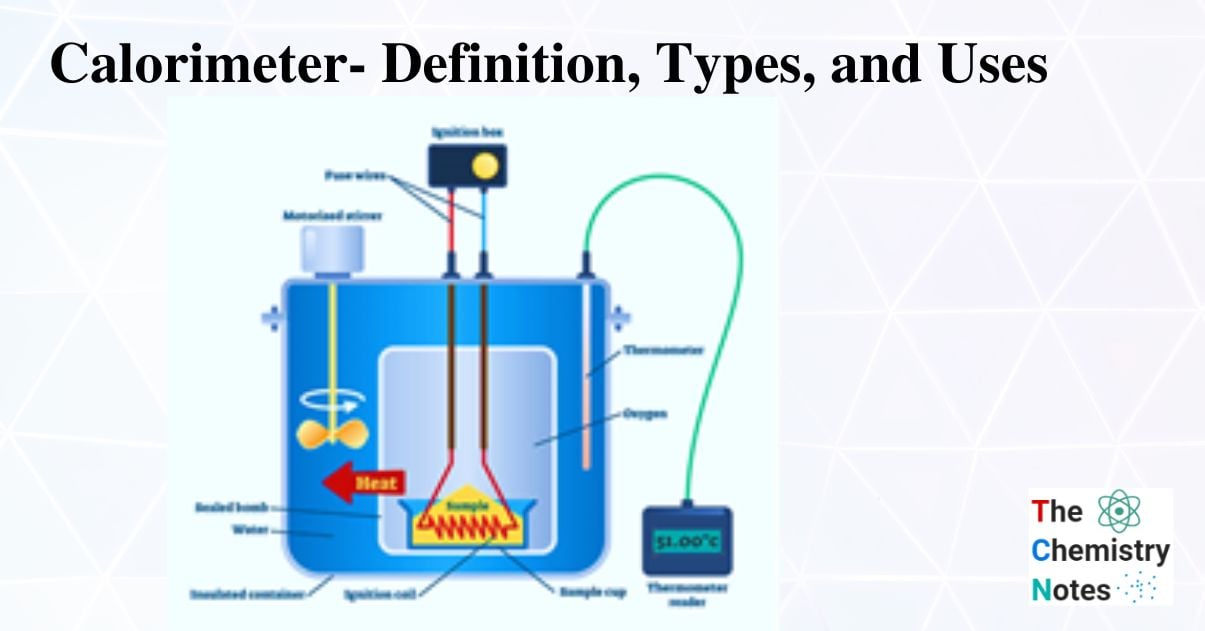
What Is Calorimeter Measured In . In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity.
from scienceinfo.com
For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
Calorimeter Definition, Types and Uses
What Is Calorimeter Measured In For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube What Is Calorimeter Measured In For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot. What Is Calorimeter Measured In.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Is Calorimeter Measured In A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a. What Is Calorimeter Measured In.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or What Is Calorimeter Measured In Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when. What Is Calorimeter Measured In.
From saylordotorg.github.io
Calorimetry What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first. What Is Calorimeter Measured In.
From www.britannica.com
Calorimeter instrument Britannica What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device. What Is Calorimeter Measured In.
From www.numerade.com
SOLVED Use the References to access important values if needed for What Is Calorimeter Measured In Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount. What Is Calorimeter Measured In.
From physique.ensc-rennes.fr
TP calorimetry What Is Calorimeter Measured In Apply the first law of thermodynamics to calorimetry. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is. What Is Calorimeter Measured In.
From www.zuniv.net
New Human Physiology Ch 20 What Is Calorimeter Measured In Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic.. What Is Calorimeter Measured In.
From solvedlib.com
260.562 g of graphite is placed in calorimeter with e… SolvedLib What Is Calorimeter Measured In In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Compare heat flow from hot to cold objects in an ideal. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeter, device for measuring the heat developed during a mechanical,. What Is Calorimeter Measured In.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube What Is Calorimeter Measured In A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. In chemistry and thermodynamics, calorimetry (from. What Is Calorimeter Measured In.
From www.thoughtco.com
Coffee Cup and Bomb Calorimetry What Is Calorimeter Measured In For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount. What Is Calorimeter Measured In.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. Calorimeter, device for measuring the. What Is Calorimeter Measured In.
From www.mdpi.com
JCM Free FullText Indirect Calorimetry in Clinical Practice What Is Calorimeter Measured In For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used. What Is Calorimeter Measured In.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry What Is Calorimeter Measured In Compare heat flow from hot to cold objects in an ideal. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. For example, when an exothermic. A calorimeter is a device. What Is Calorimeter Measured In.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is Calorimeter Measured In Compare heat flow from hot to cold objects in an ideal. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical. What Is Calorimeter Measured In.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Measured In Compare heat flow from hot to cold objects in an ideal. Apply the first law of thermodynamics to calorimetry. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring. What Is Calorimeter Measured In.
From users.highland.edu
Calorimetry What Is Calorimeter Measured In For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. Calorimeter, device. What Is Calorimeter Measured In.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The What Is Calorimeter Measured In For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. What Is Calorimeter Measured In.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 What Is Calorimeter Measured In In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. A calorimeter is. What Is Calorimeter Measured In.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry What Is Calorimeter Measured In Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. For. What Is Calorimeter Measured In.
From courses.lumenlearning.com
Calorimetry Chemistry I What Is Calorimeter Measured In For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a. What Is Calorimeter Measured In.
From www.bartleby.com
Answered In the laboratory a "coffee cup"… bartleby What Is Calorimeter Measured In For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of. What Is Calorimeter Measured In.
From www.youtube.com
Principle of Calorimetry YouTube What Is Calorimeter Measured In A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Compare heat flow. What Is Calorimeter Measured In.
From www.youtube.com
Calorimetry calculation YouTube What Is Calorimeter Measured In For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry describes a set. What Is Calorimeter Measured In.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Calorimeter Measured In In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. Calorimetry describes a set of techniques employed to measure enthalpy. What Is Calorimeter Measured In.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. A calorimeter is a device used. What Is Calorimeter Measured In.
From glossary.periodni.com
Download calorimeter.png image from What Is Calorimeter Measured In In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. What Is Calorimeter Measured In.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. Apply the first law of thermodynamics. What Is Calorimeter Measured In.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Is Calorimeter Measured In A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic.. What Is Calorimeter Measured In.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, What Is Calorimeter Measured In A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Apply the first law of thermodynamics to calorimetry. Calorimetry describes a set of techniques employed to measure enthalpy changes in. What Is Calorimeter Measured In.
From schoolbag.info
Image What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Compare heat flow from hot to cold objects in an ideal. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount. What Is Calorimeter Measured In.
From scienceinfo.com
Calorimeter Definition, Types and Uses What Is Calorimeter Measured In Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the. What Is Calorimeter Measured In.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or. What Is Calorimeter Measured In.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube What Is Calorimeter Measured In For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and. What Is Calorimeter Measured In.
From www.cbc.ca
Enter the calorimeter a chamber that measures how many calories your What Is Calorimeter Measured In Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called calorimeters. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. What Is Calorimeter Measured In.