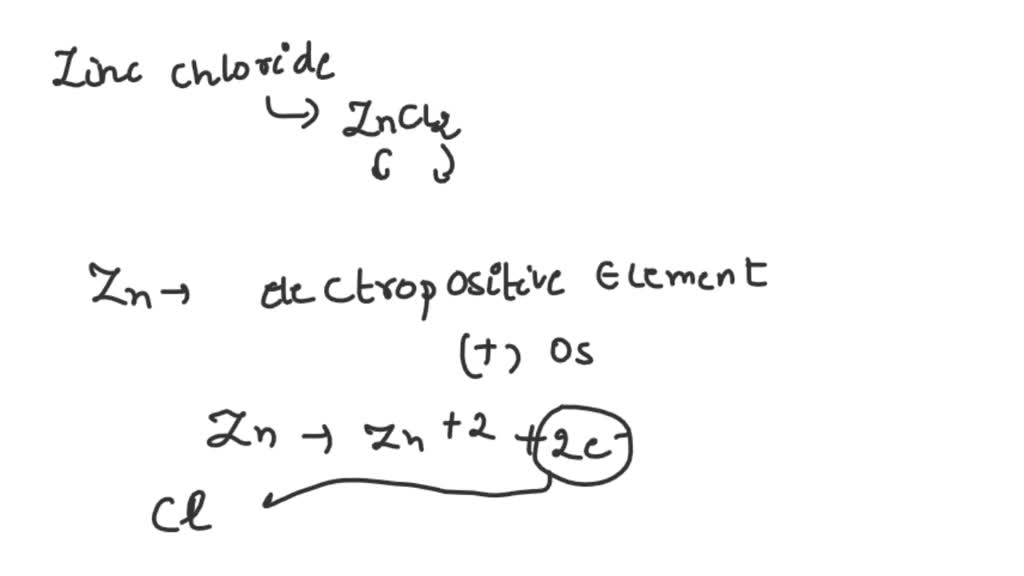
Zinc Chloride From Zinc Equation . Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zinc chloride is an inorganic compound having zinc cations and chloride anions. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. When forming an ion, a zinc atom loses its two valence electrons,. The formula for anhydrous zinc chloride. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. Zinc + hydrogen chloride = zinc chloride + dihydrogen. The formula for zinc chloride is #zncl_2#. The formula for the ionic compound zinc chloride is #zncl_2#. Zn + hcl = zncl2 + h2 is a single displacement.
from childhealthpolicy.vumc.org
Zinc chloride is an inorganic compound having zinc cations and chloride anions. When forming an ion, a zinc atom loses its two valence electrons,. Zinc + hydrogen chloride = zinc chloride + dihydrogen. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The formula for zinc chloride is #zncl_2#. Zn + hcl = zncl2 + h2 is a single displacement. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. The formula for the ionic compound zinc chloride is #zncl_2#. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4.
🌱 What is the chemical formula of zinc chloride. What is the chemical
Zinc Chloride From Zinc Equation The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zn + hcl = zncl2 + h2 is a single displacement. Zinc chloride is an inorganic compound having zinc cations and chloride anions. When forming an ion, a zinc atom loses its two valence electrons,. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. The formula for anhydrous zinc chloride. The formula for the ionic compound zinc chloride is #zncl_2#. The formula for zinc chloride is #zncl_2#. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zinc + hydrogen chloride = zinc chloride + dihydrogen. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Chloride From Zinc Equation This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The formula for the ionic compound zinc chloride is #zncl_2#. The formula for zinc chloride is #zncl_2#. The formula for anhydrous zinc chloride. When forming an ion, a zinc atom loses its two valence electrons,. The five known hydrates of zinc chloride have the general formula. Zinc Chloride From Zinc Equation.
From childhealthpolicy.vumc.org
🌱 What is the chemical formula of zinc chloride. What is the chemical Zinc Chloride From Zinc Equation The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. When forming an ion, a zinc atom loses its two valence electrons,. Zinc chloride is an inorganic compound having zinc cations and chloride anions. Zn + hcl = zncl2 +. Zinc Chloride From Zinc Equation.
From www.fishersci.co.uk
Zinc Chloride solution 0.1mol/L, Fisher Chemical Fisher Scientific Zinc Chloride From Zinc Equation The formula for the ionic compound zinc chloride is #zncl_2#. Zn + hcl = zncl2 + h2 is a single displacement. Zinc + hydrogen chloride = zinc chloride + dihydrogen. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. When forming an ion, a zinc atom loses its two valence electrons,. The five known hydrates. Zinc Chloride From Zinc Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc Chloride From Zinc Equation The formula for zinc chloride is #zncl_2#. Zinc chloride is an inorganic compound having zinc cations and chloride anions. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc + hydrogen chloride = zinc chloride + dihydrogen. When forming an ion, a zinc atom loses its two valence electrons,.. Zinc Chloride From Zinc Equation.
From jasonstark.com
Empirical Formula Lab Zinc Chloride Stark Science Zinc Chloride From Zinc Equation Zinc chloride is an inorganic compound having zinc cations and chloride anions. The formula for zinc chloride is #zncl_2#. Zn + hcl = zncl2 + h2 is a single displacement. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. The formula for the ionic compound zinc chloride is #zncl_2#. When forming. Zinc Chloride From Zinc Equation.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Zinc Chloride From Zinc Equation Zn + hcl = zncl2 + h2 is a single displacement. The formula for the ionic compound zinc chloride is #zncl_2#. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. When forming an ion, a zinc atom loses its two valence electrons,. Zinc chloride is an inorganic compound having. Zinc Chloride From Zinc Equation.
From www.numerade.com
SOLVED 'What is the empirical formula of zinc chloride? Show Zinc Chloride From Zinc Equation When forming an ion, a zinc atom loses its two valence electrons,. The formula for the ionic compound zinc chloride is #zncl_2#. The formula for anhydrous zinc chloride. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. Zinc chloride is an inorganic compound having zinc cations and chloride anions. Zinc chloride is a hygroscopic compound,. Zinc Chloride From Zinc Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Chloride From Zinc Equation The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. The formula for zinc chloride is #zncl_2#. Zinc + hydrogen chloride = zinc chloride + dihydrogen. The formula for the ionic compound zinc chloride is #zncl_2#. The formula for anhydrous. Zinc Chloride From Zinc Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Chloride From Zinc Equation The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. The formula for the ionic compound zinc chloride is #zncl_2#. Zinc +. Zinc Chloride From Zinc Equation.
From www.youtube.com
Reaction of Zinc with Hydrochloric acid Empirical formula of zinc Zinc Chloride From Zinc Equation Zinc + hydrogen chloride = zinc chloride + dihydrogen. Zinc chloride is an inorganic compound having zinc cations and chloride anions. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. The. Zinc Chloride From Zinc Equation.
From www.scribd.com
Emp Formula Zinc Chloride 0102 (2) Chloride Chlorine Zinc Chloride From Zinc Equation The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. The formula for zinc chloride is #zncl_2#. The formula for the ionic compound zinc chloride is #zncl_2#. When forming an ion, a zinc atom loses its two valence electrons,. Zn. Zinc Chloride From Zinc Equation.
From www.youtube.com
How to Write zinc chloride Molecular formula Chemical formula of zinc Zinc Chloride From Zinc Equation Zinc + hydrogen chloride = zinc chloride + dihydrogen. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zn + hcl = zncl2 + h2 is a single displacement. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n. Zinc Chloride From Zinc Equation.
From www.pakistanchemical.com
Zinc Chloride PAKISTAN CHEMICAL Zinc Chloride From Zinc Equation Zinc chloride is an inorganic compound having zinc cations and chloride anions. The formula for zinc chloride is #zncl_2#. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zn + hcl = zncl2 + h2 is a single displacement. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride. Zinc Chloride From Zinc Equation.
From childhealthpolicy.vumc.org
🌱 What is the chemical formula of zinc chloride. What is the chemical Zinc Chloride From Zinc Equation The formula for zinc chloride is #zncl_2#. The formula for the ionic compound zinc chloride is #zncl_2#. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zinc chloride is a hygroscopic compound, that will absorb water from air to. Zinc Chloride From Zinc Equation.
From www.sunshinetrading.co
Zinc Chloride Sunshine Trading Company Zinc Chloride From Zinc Equation The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zinc chloride is an inorganic compound having zinc cations and chloride anions. The formula for the ionic compound zinc chloride is #zncl_2#. This represents a ratio of one zinc ion. Zinc Chloride From Zinc Equation.
From www.chegg.com
Solved A. Zinc Chloride 1. Mass of evaporating dish and zinc Zinc Chloride From Zinc Equation This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The formula for zinc chloride is #zncl_2#. Zinc + hydrogen chloride = zinc chloride + dihydrogen. The formula for the ionic compound zinc chloride is #zncl_2#. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. The formula. Zinc Chloride From Zinc Equation.
From express.adobe.com
Zinc and Copper Chloride Zinc Chloride From Zinc Equation Zn + hcl = zncl2 + h2 is a single displacement. Zinc chloride is an inorganic compound having zinc cations and chloride anions. The formula for anhydrous zinc chloride. The formula for the ionic compound zinc chloride is #zncl_2#. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zinc + hydrogen. Zinc Chloride From Zinc Equation.
From www.researchgate.net
(PDF) The Structure of Zinc Chloride Complexes in Aqueous Solution Zinc Chloride From Zinc Equation This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The formula for zinc chloride is #zncl_2#. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zn + hcl = zncl2 + h2 is a single displacement. The five known hydrates of zinc chloride have the general. Zinc Chloride From Zinc Equation.
From www.pw.live
Zinc Chloride Formula Zinc Chloride From Zinc Equation The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. The formula for anhydrous zinc chloride. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. The formula for zinc. Zinc Chloride From Zinc Equation.
From brainly.in
write molecular formula of zinc chloride using crisscross method Zinc Chloride From Zinc Equation This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The formula for anhydrous zinc chloride. When forming an ion, a zinc atom loses its two valence electrons,. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chloride is a hygroscopic compound, that will. Zinc Chloride From Zinc Equation.
From www.chegg.com
Solved REPORT SHEET Chemical Formulas A, Zinc Chloride 1. Zinc Chloride From Zinc Equation When forming an ion, a zinc atom loses its two valence electrons,. Zinc chloride is an inorganic compound having zinc cations and chloride anions. Zinc + hydrogen chloride = zinc chloride + dihydrogen. The formula for the ionic compound zinc chloride is #zncl_2#. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The formula for. Zinc Chloride From Zinc Equation.
From www.chegg.com
Solved REPORT SHEET EXP Chemical Formulas 5 A. Zinc Chloride Zinc Chloride From Zinc Equation The formula for anhydrous zinc chloride. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. The formula for the ionic compound zinc chloride is #zncl_2#. When forming an ion, a zinc atom loses its two valence electrons,. Zn + hcl = zncl2 + h2 is a single displacement. Zinc. Zinc Chloride From Zinc Equation.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride From Zinc Equation Zinc chloride is an inorganic compound having zinc cations and chloride anions. When forming an ion, a zinc atom loses its two valence electrons,. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zn + hcl = zncl2 +. Zinc Chloride From Zinc Equation.
From askfilo.com
zinc chloride(IV). 30. Correct formula of tetraamminechloridonitroplatinu.. Zinc Chloride From Zinc Equation This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. Zinc chloride is an inorganic compound having zinc cations and chloride anions. The formula for anhydrous zinc chloride. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zn + hcl = zncl2 + h2 is a single. Zinc Chloride From Zinc Equation.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride From Zinc Equation When forming an ion, a zinc atom loses its two valence electrons,. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. Zinc chloride is an inorganic compound having zinc cations and chloride anions. Zn + hcl = zncl2 + h2 is a single displacement. The five known hydrates of zinc chloride have the general formula. Zinc Chloride From Zinc Equation.
From camachem.com
What is Zinc Chloride and How to Buy Zinc Chloride? Zinc Chloride From Zinc Equation The formula for the ionic compound zinc chloride is #zncl_2#. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. When forming an ion, a zinc atom loses its two valence electrons,. This represents a ratio of one zinc ion. Zinc Chloride From Zinc Equation.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Chloride From Zinc Equation Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc + hydrogen chloride = zinc chloride + dihydrogen. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. The formula for anhydrous zinc chloride. The five known hydrates of zinc chloride have the general formula. Zinc Chloride From Zinc Equation.
From www.coursehero.com
[Solved] how do you find the theoretical mass of the product zinc Zinc Chloride From Zinc Equation Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zinc + hydrogen chloride = zinc chloride + dihydrogen. Zn + hcl = zncl2 + h2 is a single displacement. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. Zinc chloride, represented by the chemical formula zncl. Zinc Chloride From Zinc Equation.
From www.toppr.com
How will you obtain zinc chloride from zinc sulphate? Zinc Chloride From Zinc Equation Zinc + hydrogen chloride = zinc chloride + dihydrogen. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zn + hcl = zncl2 + h2 is a single displacement. When forming an ion, a zinc atom loses its two valence electrons,. Zinc chloride, represented by the chemical formula zncl 2, is. Zinc Chloride From Zinc Equation.
From www.flinnsci.com
Flinn Chemicals, Zinc Chloride Zinc Chloride From Zinc Equation Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. When forming an ion, a zinc atom loses its two valence electrons,. The formula for the ionic compound zinc chloride is #zncl_2#. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. Zn + hcl = zncl2 +. Zinc Chloride From Zinc Equation.
From www.indiamart.com
Zinc Chloride Solution Technical Grade Zinc Chloride Solution Zinc Chloride From Zinc Equation Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zinc + hydrogen chloride = zinc chloride + dihydrogen. The five known hydrates of zinc chloride have the general formula zncl 2. Zinc Chloride From Zinc Equation.
From gionioblo.blob.core.windows.net
Zinc Chloride Metal Equation at Randy Edwards blog Zinc Chloride From Zinc Equation The formula for zinc chloride is #zncl_2#. Zinc + hydrogen chloride = zinc chloride + dihydrogen. This represents a ratio of one zinc ion #zn^(2+)#, to two chloride ions cl−. Zinc chloride is an inorganic compound having zinc cations and chloride anions. The formula for anhydrous zinc chloride. When forming an ion, a zinc atom loses its two valence electrons,.. Zinc Chloride From Zinc Equation.
From www.carolina.com
Zinc Chloride, Reagent Grade, 500 g Zinc Chloride From Zinc Equation The formula for anhydrous zinc chloride. The formula for zinc chloride is #zncl_2#. Zinc chloride is an inorganic compound having zinc cations and chloride anions. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. When forming an ion, a. Zinc Chloride From Zinc Equation.
From studylib.net
Determine the Chemical Formula for Zinc Chloride Zinc Chloride From Zinc Equation Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zn + hcl = zncl2 + h2 is a single displacement. This. Zinc Chloride From Zinc Equation.
From www.dreamstime.com
Molecular Formula of Zinc Chloride Stock Illustration Illustration of Zinc Chloride From Zinc Equation The formula for zinc chloride is #zncl_2#. Zinc chloride, represented by the chemical formula zncl 2, is a white crystalline solid that is highly soluble in water. The formula for the ionic compound zinc chloride is #zncl_2#. Zinc chloride is a hygroscopic compound, that will absorb water from air to form at least 5 hydrates. Zinc chloride is an inorganic. Zinc Chloride From Zinc Equation.