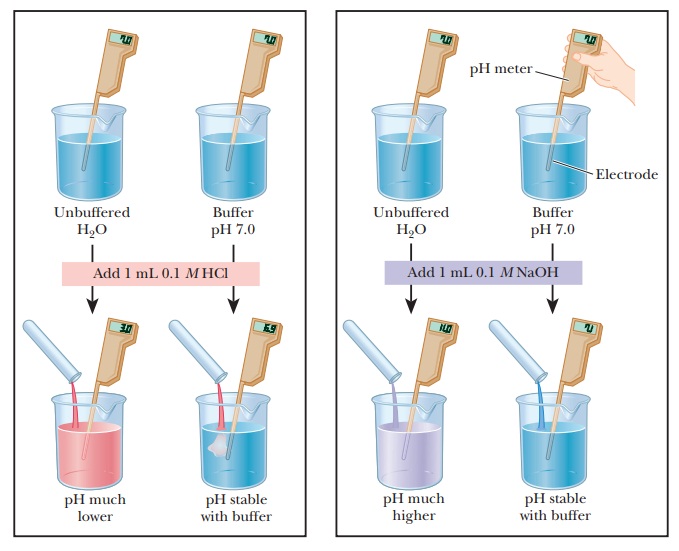
Buffer Solution Definition In Biology . Buffers, ph, acids, and bases. In this way, a biological buffer. To review, buffer solutions contain a weak acid and its conjugate base. They have maximal buffering capacity at a ph = pka of the. You have probably used litmus paper, paper. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The ph of a solution is a measure of its acidity or alkalinity. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.
from www.brainkart.com
The ph of a solution is a measure of its acidity or alkalinity. To review, buffer solutions contain a weak acid and its conjugate base. In this way, a biological buffer. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Buffers, ph, acids, and bases. You have probably used litmus paper, paper. They have maximal buffering capacity at a ph = pka of the. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional.
Buffers
Buffer Solution Definition In Biology Buffers, ph, acids, and bases. They have maximal buffering capacity at a ph = pka of the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. To review, buffer solutions contain a weak acid and its conjugate base. You have probably used litmus paper, paper. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. In this way, a biological buffer. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. Buffers, ph, acids, and bases. The ph of a solution is a measure of its acidity or alkalinity.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Buffer Solution Definition In Biology The ph of a solution is a measure of its acidity or alkalinity. Buffers, ph, acids, and bases. They have maximal buffering capacity at a ph = pka of the. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. To review, buffer solutions contain a. Buffer Solution Definition In Biology.
From www.pinterest.com
Pin on Download Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. They have maximal buffering capacity at a ph = pka of the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. You have probably used litmus paper, paper. In this way, a biological buffer. A buffer is a solution containing acid and. Buffer Solution Definition In Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 Buffer Solution Definition In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Buffers, ph, acids, and bases. In this way, a biological buffer. You have probably used litmus paper, paper. To review, buffer solutions contain a weak acid and its conjugate base. A buffer is a solution containing acid and a proportionate amount of conjugate base capable. Buffer Solution Definition In Biology.
From www.youtube.com
The Mechanism of Buffer Solutions Clearly Explained YouTube Buffer Solution Definition In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological buffer. To review, buffer solutions contain a weak acid and its conjugate base. You have probably used litmus paper, paper. The ph of a solution is a measure of its acidity or alkalinity. A buffer is a solution containing acid. Buffer Solution Definition In Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. You have probably used litmus paper, paper. They have maximal buffering capacity at a ph = pka of the. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A biological buffer is an. Buffer Solution Definition In Biology.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Definition In Biology In this way, a biological buffer. They have maximal buffering capacity at a ph = pka of the. Buffers, ph, acids, and bases. The ph of a solution is a measure of its acidity or alkalinity. To review, buffer solutions contain a weak acid and its conjugate base. You have probably used litmus paper, paper. The purpose of a buffer. Buffer Solution Definition In Biology.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Definition In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. You have probably used litmus paper, paper. The ph of a solution is a measure of its acidity or alkalinity. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A. Buffer Solution Definition In Biology.
From www.studypool.com
SOLUTION Buffer solution types, definition and functions Studypool Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. The ph of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper, paper. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The purpose of a buffer in a biological system is to maintain intracellular. Buffer Solution Definition In Biology.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation ID2948821 Buffer Solution Definition In Biology They have maximal buffering capacity at a ph = pka of the. Buffers, ph, acids, and bases. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. You have probably used litmus paper, paper. The ph of a solution is a measure of its. Buffer Solution Definition In Biology.
From www.pinterest.com
Buffer Buffer solution, Easy science, Flashcards Buffer Solution Definition In Biology You have probably used litmus paper, paper. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. They have maximal buffering capacity at a ph = pka of the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this. Buffer Solution Definition In Biology.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Buffer Solution Definition In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological buffer. You have probably used litmus paper, paper. They have maximal buffering capacity at a ph = pka of the. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph. Buffer Solution Definition In Biology.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses Buffer Solution Definition In Biology You have probably used litmus paper, paper. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. To review, buffer solutions contain a weak acid and its conjugate base.. Buffer Solution Definition In Biology.
From www.slideserve.com
PPT BUFFER SOLUTIONS A guide for A level students PowerPoint Buffer Solution Definition In Biology The ph of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper, paper. In this way, a biological buffer. Buffers, ph, acids, and bases. They have maximal buffering capacity at a ph = pka of the. To review, buffer solutions contain a weak acid and its conjugate base. The purpose of a buffer. Buffer Solution Definition In Biology.
From studylib.net
Buffer Solutions Buffer Solution Definition In Biology A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. To review, buffer solutions contain a weak acid and its conjugate base. Buffers, ph, acids, and bases. You have probably used litmus paper, paper. The purpose of a buffer in a biological system is. Buffer Solution Definition In Biology.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and. Buffer Solution Definition In Biology.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Buffer Solution Definition In Biology Buffers, ph, acids, and bases. You have probably used litmus paper, paper. In this way, a biological buffer. The ph of a solution is a measure of its acidity or alkalinity. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A buffer is a solution. Buffer Solution Definition In Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. In this way, a biological buffer. They have maximal buffering capacity at a ph = pka of the. You have probably used litmus paper, paper. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small. Buffer Solution Definition In Biology.
From golifescience.com
Buffer Solution definition, 4 Types and Basic Calculations Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. You have probably used litmus paper, paper. The ph of a solution is a measure of its acidity or alkalinity. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. In this. Buffer Solution Definition In Biology.
From www.osmosis.org
Making buffer solutions Osmosis Buffer Solution Definition In Biology In this way, a biological buffer. To review, buffer solutions contain a weak acid and its conjugate base. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The ph of a solution is a measure of its acidity or alkalinity. A buffer is a solution. Buffer Solution Definition In Biology.
From fr.slideshare.net
Buffer solution Buffer Solution Definition In Biology The ph of a solution is a measure of its acidity or alkalinity. They have maximal buffering capacity at a ph = pka of the. Buffers, ph, acids, and bases. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. You have probably used litmus paper,. Buffer Solution Definition In Biology.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Solution Definition In Biology The ph of a solution is a measure of its acidity or alkalinity. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffers, ph, acids, and bases. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a. Buffer Solution Definition In Biology.
From www.slideserve.com
PPT Lecture 2 Dr. Kumar Aidbase balanceSalivary Buffering Buffer Solution Definition In Biology A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. In this way, a biological buffer. You have probably used litmus paper, paper. To review, buffer solutions contain a weak acid and its conjugate base. The ph of a solution is a measure of. Buffer Solution Definition In Biology.
From www.expii.com
Buffers — Definition & Overview Expii Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. You have probably used litmus paper, paper. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffers, ph, acids, and bases. In this way, a biological buffer. A buffer is a solution containing. Buffer Solution Definition In Biology.
From avantecnica.qualitypoolsboulder.com
Buffer Solution Definition, Types, Formula, Examples, and FAQs Buffer Solution Definition In Biology They have maximal buffering capacity at a ph = pka of the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. To review, buffer solutions contain a weak acid and its conjugate base. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when. Buffer Solution Definition In Biology.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 Buffer Solution Definition In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. To review, buffer solutions contain a weak acid and its conjugate base. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. They have maximal buffering capacity at a ph =. Buffer Solution Definition In Biology.
From loeukppln.blob.core.windows.net
Buffers Ap Biology at Venessa Baird blog Buffer Solution Definition In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The ph of a solution is a measure of its acidity or alkalinity. They have maximal buffering capacity at a ph = pka of the. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Buffer Solution Definition In Biology.
From www.slideshare.net
Buffer system Buffer Solution Definition In Biology To review, buffer solutions contain a weak acid and its conjugate base. Buffers, ph, acids, and bases. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological buffer. They have maximal buffering capacity at a ph = pka of the. The ph of a solution is a measure of its. Buffer Solution Definition In Biology.
From www.toppr.com
Buffer Solutions Definition, Types, Preparation, Examples and Videos Buffer Solution Definition In Biology A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. They have maximal buffering capacity at a ph = pka of the. In this way, a biological buffer. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph. Buffer Solution Definition In Biology.
From www.pdfprof.com
PDF examples of buffer solutions PDF Télécharger Download Buffer Solution Definition In Biology You have probably used litmus paper, paper. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. Buffers, ph, acids, and bases. To review, buffer solutions contain a weak acid and its conjugate base. A biological buffer is an organic substance that has a. Buffer Solution Definition In Biology.
From www.brainkart.com
Buffers Buffer Solution Definition In Biology The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. You have probably used litmus paper, paper. In this way, a biological buffer. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Buffers, ph, acids, and bases. They have maximal. Buffer Solution Definition In Biology.
From www.studypool.com
SOLUTION Buffer solution types, definition and functions Studypool Buffer Solution Definition In Biology A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. In this way, a biological buffer. To review, buffer solutions contain a weak acid and its conjugate base. They have maximal buffering capacity at a ph = pka of the. The purpose of a. Buffer Solution Definition In Biology.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution Definition In Biology A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. In this way, a biological buffer. You have probably used litmus paper, paper. Buffers, ph, acids, and bases. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The. Buffer Solution Definition In Biology.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer Solution Definition In Biology In this way, a biological buffer. To review, buffer solutions contain a weak acid and its conjugate base. A buffer is a solution containing acid and a proportionate amount of conjugate base capable of maintaining a stable ph when a small amount of additional. Buffers, ph, acids, and bases. The purpose of a buffer in a biological system is to. Buffer Solution Definition In Biology.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk Buffer Solution Definition In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. You have probably used litmus paper, paper. The ph of a solution is a measure of its acidity or alkalinity. In this way, a biological buffer. They have maximal buffering capacity at a ph = pka of the. The purpose of a buffer in a. Buffer Solution Definition In Biology.