Acetic Acid Glacial Molarity . Molarity refers to the number of moles of the. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic acid is 1.049 grams at 25°c. Glacial acetic acid is acetic acid that contains little to no water. In the video we use the equation molarity =. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. It is called “glacial” because it freezes. In other words, it is a purified or concentrated form of anhydrous acetic acid. It is widely used in organic syntheses, in the manufacture. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Acetic acid (acoh) can be used as: Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml.
from www.chegg.com
In other words, it is a purified or concentrated form of anhydrous acetic acid. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. Molarity refers to the number of moles of the. Acetic acid (acoh) can be used as: A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). In the video we use the equation molarity =. It is widely used in organic syntheses, in the manufacture. Glacial acetic acid is acetic acid that contains little to no water. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1.
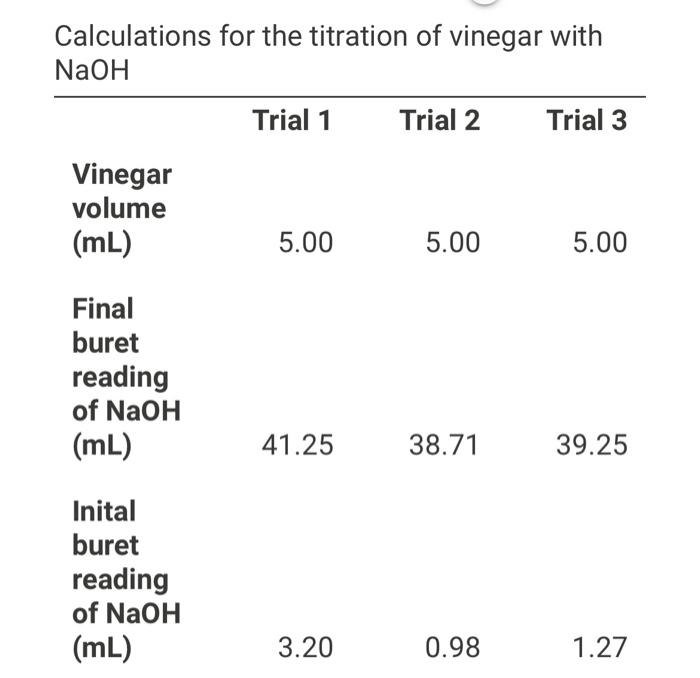
Solved (1pts) Average molarity of acetic acid (M) (1pts)
Acetic Acid Glacial Molarity Acetic acid (acoh) can be used as: A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. Acetic acid (acoh) can be used as: Molarity refers to the number of moles of the. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. It is called “glacial” because it freezes. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic acid is 1.049 grams at 25°c. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). Glacial acetic acid is acetic acid that contains little to no water. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. In the video we use the equation molarity =. It is widely used in organic syntheses, in the manufacture. In other words, it is a purified or concentrated form of anhydrous acetic acid.
From www.indiamart.com
Fisher Scientific Acetic Acid Glacial, For Laboratory, Bottles at Rs Acetic Acid Glacial Molarity In other words, it is a purified or concentrated form of anhydrous acetic acid. It is called “glacial” because it freezes. Molarity refers to the number of moles of the. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. The. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). Glacial acetic acid is acetic acid that contains little to no water. In the video we use the equation molarity =. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity Glacial acetic acid is acetic acid that contains little to no water. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7. Acetic Acid Glacial Molarity.
From chemfulfillment.com
Glacial Acetic Acid 99 Pure Ethanoic Acid Chemfulfill Chemical Acetic Acid Glacial Molarity A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. It is widely used in organic syntheses, in the manufacture. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. Glacial acetic acid. Acetic Acid Glacial Molarity.
From www.fishersci.com
Acetic Acid (100, Glacial), Anhydrous, EMSURE MilliporeSigma, Quantity Acetic Acid Glacial Molarity It is widely used in organic syntheses, in the manufacture. Acetic acid (acoh) can be used as: It is called “glacial” because it freezes. In other words, it is a purified or concentrated form of anhydrous acetic acid. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. A 99.7% (w/w) glacial acetic acid means that 100. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. In other words, it is a purified or concentrated form of anhydrous acetic acid. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic acid is 1.049 grams at 25°c. It is widely used in organic syntheses, in the manufacture. The density of glacial acetic. Acetic Acid Glacial Molarity.
From www.indiamart.com
Acetic Acid Glacial LR 500ml at Rs 400 in Hyderabad ID 18687770273 Acetic Acid Glacial Molarity Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. Molarity refers to the number of moles of the. Acetic acid (acoh) can be used as: A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity It is widely used in organic syntheses, in the manufacture. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Molarity refers to the number of moles of the. A 99.7% (w/w) glacial acetic acid means that. Acetic Acid Glacial Molarity.
From acetic-acid.net
Glacial Acetic Acid Uses Comprehensive Overview Acetic Acid Glacial Molarity It is widely used in organic syntheses, in the manufacture. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Acetic acid (acoh) can be used as: Concentrated, or glacial, acetic acid is pure acetic acid and has. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic acid is 1.049 grams at 25°c. Acetic acid (acoh) can be used as: The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. It is called “glacial” because. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Molarity refers to the number of moles of the. It is widely used in organic syntheses, in the manufacture. In the video we use the equation molarity =.. Acetic Acid Glacial Molarity.
From www.thistlescientific.co.uk
Glacial acetic acidAnalytical Grade Thistle Scientific Acetic Acid Glacial Molarity A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). In other words, it is a purified or concentrated form of anhydrous acetic acid. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. In the video we use the equation molarity =. Glacial acetic acid is. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity In other words, it is a purified or concentrated form of anhydrous acetic acid. It is called “glacial” because it freezes. Molarity refers to the number of moles of the. Glacial acetic acid is acetic acid that contains little to no water. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity In the video we use the equation molarity =. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. In other. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). It is widely used in organic syntheses, in the manufacture. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. Molarity refers to the number of moles of the. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity In other words, it is a purified or concentrated form of anhydrous acetic acid. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). It is widely used in organic syntheses, in the manufacture. The density. Acetic Acid Glacial Molarity.
From ar.inspiredpencil.com
Glacial Acetic Acid Structure Acetic Acid Glacial Molarity A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Molarity refers to the number of moles of the. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. It is called “glacial” because it freezes. Concentrated, or glacial, acetic acid is pure acetic acid and has. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. Glacial acetic acid is acetic acid that contains little to no water. It is widely used in organic syntheses, in the manufacture. In the video we use the. Acetic Acid Glacial Molarity.
From www.numerade.com
SOLVED Pure acetic acid, known as glacial acetic acid, is a liquid Acetic Acid Glacial Molarity The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic acid is 1.049 grams at 25°c. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Acetic acid (acoh) can be used as: Molarity refers to the number. Acetic Acid Glacial Molarity.
From www.sbcscientific.com
Acetic Acid Glacial(Analysis, Fisher) Acetic Acid Glacial Molarity In the video we use the equation molarity =. It is widely used in organic syntheses, in the manufacture. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. In the video we use the equation molarity =. Glacial acetic acid is acetic acid that contains little to no water. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic. Acetic Acid Glacial Molarity.
From ar.inspiredpencil.com
Glacial Acetic Acid Structure Acetic Acid Glacial Molarity A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. In the video we use the equation molarity =. It is widely used in organic syntheses, in the manufacture. Glacial acetic acid is acetic acid that contains little to no water. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml.. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity Molarity refers to the number of moles of the. It is widely used in organic syntheses, in the manufacture. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. Acetic acid (acoh) can be used as: The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the. Acetic Acid Glacial Molarity.
From www.laboratorynotes.com
Molarity of Glacial Acetic Acid (99.7, w/w, CH3COOH) Laboratory Notes Acetic Acid Glacial Molarity Glacial acetic acid is acetic acid that contains little to no water. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Acetic acid (acoh) can be used as: It is called “glacial” because it freezes. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity It is widely used in organic syntheses, in the manufacture. It is called “glacial” because it freezes. A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). Glacial acetic acid is acetic acid that contains little to no water. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g. Acetic Acid Glacial Molarity.
From www.bartleby.com
Answered The Molarity of Acetic Acid in Vinegar… bartleby Acetic Acid Glacial Molarity Glacial acetic acid is acetic acid that contains little to no water. In other words, it is a purified or concentrated form of anhydrous acetic acid. Molarity refers to the number of moles of the. Acetic acid (acoh) can be used as: The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity A quick video on how to calculate the molarity of glacial acetic acid (ch3cooh). Molarity refers to the number of moles of the. It is widely used in organic syntheses, in the manufacture. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. In other words, it is a purified or. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity Acetic acid (acoh) can be used as: Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic acid is 1.049 grams at 25°c. It is called “glacial” because it freezes. A. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity Molarity refers to the number of moles of the. A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions,. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. In other words, it is a purified or concentrated form of anhydrous acetic acid. A quick video on how to calculate the. Acetic Acid Glacial Molarity.
From www.grainger.com
64197, 60.05, Acetic Acid, Glacial 30UC68A128002500.0 Grainger Acetic Acid Glacial Molarity The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Glacial acetic acid is acetic acid that contains little to no water. Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. It is widely used in organic syntheses, in the manufacture. In other words,. Acetic Acid Glacial Molarity.
From safrole.com
Acetic Acid Properties, Synthesis and Application Safrole Acetic Acid Glacial Molarity The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1 ml of glacial acetic acid is 1.049 grams at 25°c. Molarity refers to the number of moles of the. It is widely used in organic syntheses, in the manufacture. Concentrated, or glacial, acetic acid is pure acetic acid and has a. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. In the video we use the equation molarity =. Glacial acetic acid is acetic acid that contains little to no water. The density of glacial. Acetic Acid Glacial Molarity.
From
Acetic Acid Glacial Molarity The density of glacial acetic acid is 1.049 g/ml at 25°c which means that the weight of the 1. Acetic acid (acoh) can be used as: In the video we use the equation molarity =. Glacial acetic acid is acetic acid that contains little to no water. Concentrated, or glacial, acetic acid is pure acetic acid and has a density. Acetic Acid Glacial Molarity.
From www.sciencephoto.com
Ethanoic acid, glacial and liquid Stock Image C018/0081 Science Acetic Acid Glacial Molarity Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/ml. Acetic acid (acoh) can be used as: Glacial acetic acid is acetic acid that contains little to no water. A 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. In other words, it is. Acetic Acid Glacial Molarity.