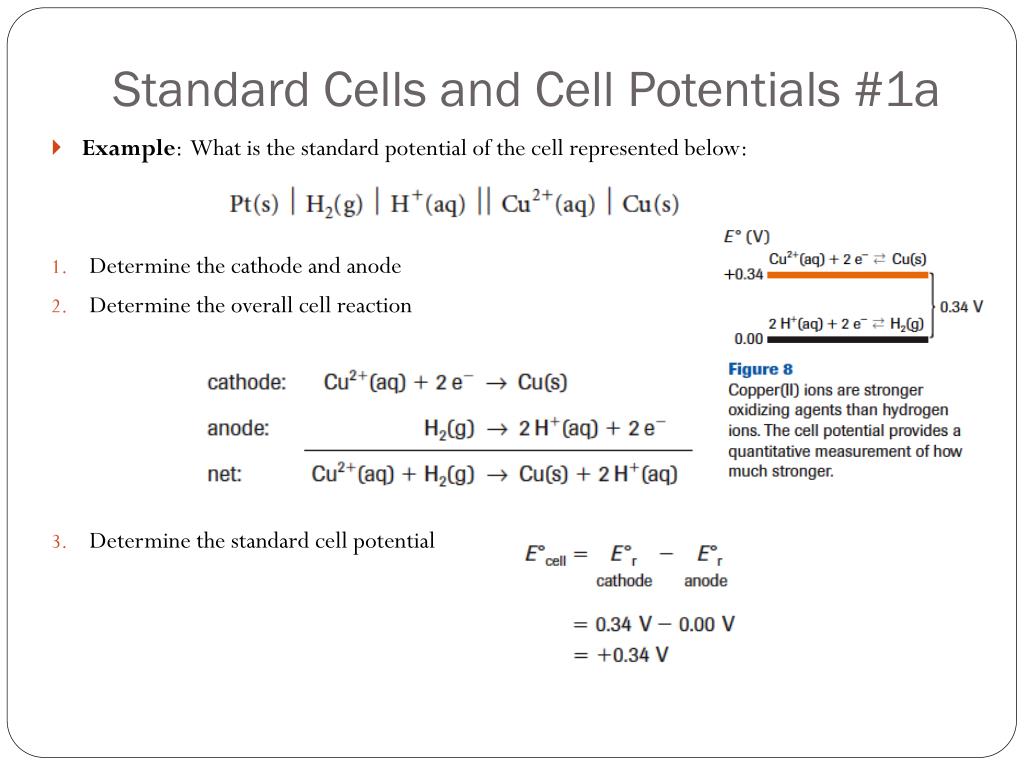
Standard Cell Potential Of Silver . 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. The galvanic cell potential results from the voltage difference of a pair of electrodes. It is not possible to. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. standard cell potential.
from www.slideserve.com
the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. It is not possible to. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The galvanic cell potential results from the voltage difference of a pair of electrodes.
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation, free download ID5480109
Standard Cell Potential Of Silver the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. The galvanic cell potential results from the voltage difference of a pair of electrodes. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. standard cell potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. It is not possible to.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Cell Potential Of Silver 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The galvanic cell potential results from the voltage difference of a pair of electrodes. the cell potential for a voltaic cell under standard conditions. Standard Cell Potential Of Silver.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Learning Inc Standard Cell Potential Of Silver 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. standard cell potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Of Silver.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Of Silver standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. It is not possible to. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. . Standard Cell Potential Of Silver.
From users.highland.edu
Standard Potentials Standard Cell Potential Of Silver the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. It is not possible to. The galvanic cell potential results from the. Standard Cell Potential Of Silver.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Cell Potential Of Silver standard cell potential. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. The galvanic cell potential results from the voltage difference of a. Standard Cell Potential Of Silver.
From www.numerade.com
SOLVED What is the standard cell potential at 25 °C for a cell that consists of a silver Standard Cell Potential Of Silver The galvanic cell potential results from the voltage difference of a pair of electrodes. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. standard cell potential. 372. Standard Cell Potential Of Silver.
From byjus.com
A copper_ silver cell is set up. The copper ion concentration is 0.10 M. The concentration of Standard Cell Potential Of Silver the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the. Standard Cell Potential Of Silver.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Standard Cell Potential Of Silver the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. The galvanic cell potential results from the voltage difference of a pair of electrodes. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. 372 rows the data below tabulates standard electrode potentials. Standard Cell Potential Of Silver.
From 2012books.lardbucket.org
Standard Potentials Standard Cell Potential Of Silver The galvanic cell potential results from the voltage difference of a pair of electrodes. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. It is not possible to. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. 372 rows the data. Standard Cell Potential Of Silver.
From exohmihef.blob.core.windows.net
Standard Electrode Potential Meaning at Francis McQuay blog Standard Cell Potential Of Silver 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. standard cell potential. It is not possible to. the potential of the cell under standard conditions (1 m for. Standard Cell Potential Of Silver.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in... Download Scientific Diagram Standard Cell Potential Of Silver The galvanic cell potential results from the voltage difference of a pair of electrodes. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. the cell potential for a voltaic cell under standard conditions can be calculated from. Standard Cell Potential Of Silver.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3975392 Standard Cell Potential Of Silver the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the cell potential for a voltaic cell under standard conditions can. Standard Cell Potential Of Silver.
From www.youtube.com
Electrochemistry 05 Calculating Standard Cell Potentials YouTube Standard Cell Potential Of Silver standard cell potential. The galvanic cell potential results from the voltage difference of a pair of electrodes. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 372 rows the data below tabulates standard electrode potentials (e. Standard Cell Potential Of Silver.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Cell Potential Of Silver 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases,. Standard Cell Potential Of Silver.
From quizspattering.z21.web.core.windows.net
How To Calculate Standard Potential Standard Cell Potential Of Silver standard cell potential. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The galvanic cell potential results from the voltage difference of a pair of electrodes. 372 rows the data below tabulates standard electrode. Standard Cell Potential Of Silver.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Of Silver It is not possible to. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. The. Standard Cell Potential Of Silver.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Standard Cell Potential Standard Cell Potential Of Silver It is not possible to. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that uses the. Standard Cell Potential Of Silver.
From exopsmjjd.blob.core.windows.net
Standard Cell Potential Variable at Corey Eberhardt blog Standard Cell Potential Of Silver It is not possible to. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. standard cell potential. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. The. Standard Cell Potential Of Silver.
From www.chegg.com
Solved Calculate the standard cell potential for a voltaic Standard Cell Potential Of Silver The galvanic cell potential results from the voltage difference of a pair of electrodes. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. 42 rows calculate the. Standard Cell Potential Of Silver.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation, free download ID5480109 Standard Cell Potential Of Silver the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The galvanic cell potential results from the voltage difference of a pair of. Standard Cell Potential Of Silver.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium/Silver Galvanic Cell Nagwa Standard Cell Potential Of Silver 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. The galvanic cell. Standard Cell Potential Of Silver.
From dokumen.tips
(PDF) Standard Potentials of HydrogenSilverSilver Chloride Cells in Ethylene Glycol Solutions Standard Cell Potential Of Silver standard cell potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. The galvanic cell potential results from the voltage difference of a. Standard Cell Potential Of Silver.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Standard Cell Potential Of Silver The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. standard cell potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. the cell potential for a voltaic cell under standard conditions can be calculated from the. Standard Cell Potential Of Silver.
From www.slideserve.com
PPT Chemistry 1011 PowerPoint Presentation, free download ID5404690 Standard Cell Potential Of Silver standard cell potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The galvanic cell potential results from the voltage difference of a pair of electrodes. The standard cell. Standard Cell Potential Of Silver.
From www.chegg.com
Solved 5. What is the standard cell potential (E cel) for Standard Cell Potential Of Silver 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. standard cell potential. It is not possible to. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes,. Standard Cell Potential Of Silver.
From thechemistrynotes.com
Cell Potential & Standard Cell Potential Standard Cell Potential Of Silver standard cell potential. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other. Standard Cell Potential Of Silver.
From www.sliderbase.com
Electrochemical Terminology Standard Cell Potential Of Silver 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The galvanic cell potential results from the voltage difference of a pair of electrodes. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a. Standard Cell Potential Of Silver.
From www.scribd.com
Standard Cell Potential Table PDF Standard Cell Potential Of Silver 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. standard cell potential. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. The standard cell potential (\(e^o_{cell}\)) is the difference. Standard Cell Potential Of Silver.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Of Silver the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. The galvanic cell potential results from the voltage difference of a pair of electrodes. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell. Standard Cell Potential Of Silver.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Of Silver It is not possible to. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The galvanic cell potential results from the voltage difference of a pair of electrodes. the cell potential for. Standard Cell Potential Of Silver.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Cell Potential Of Silver standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. It is not possible to. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The galvanic cell potential results from the voltage difference of a pair of electrodes. the cell potential for a. Standard Cell Potential Of Silver.
From www.chegg.com
Solved Use the half reactions below to produce a voltaic Standard Cell Potential Of Silver The galvanic cell potential results from the voltage difference of a pair of electrodes. standard cell potential. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. the potential of the cell under standard conditions. Standard Cell Potential Of Silver.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Of Silver 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. It is not possible to. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the cell potential for a voltaic cell under standard conditions can be calculated from the. Standard Cell Potential Of Silver.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID5581588 Standard Cell Potential Of Silver the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. It is not possible to. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms.. Standard Cell Potential Of Silver.
From www.chegg.com
Solved Calculate the standard cell potential of the Standard Cell Potential Of Silver the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The galvanic cell potential results from the voltage difference of a pair. Standard Cell Potential Of Silver.