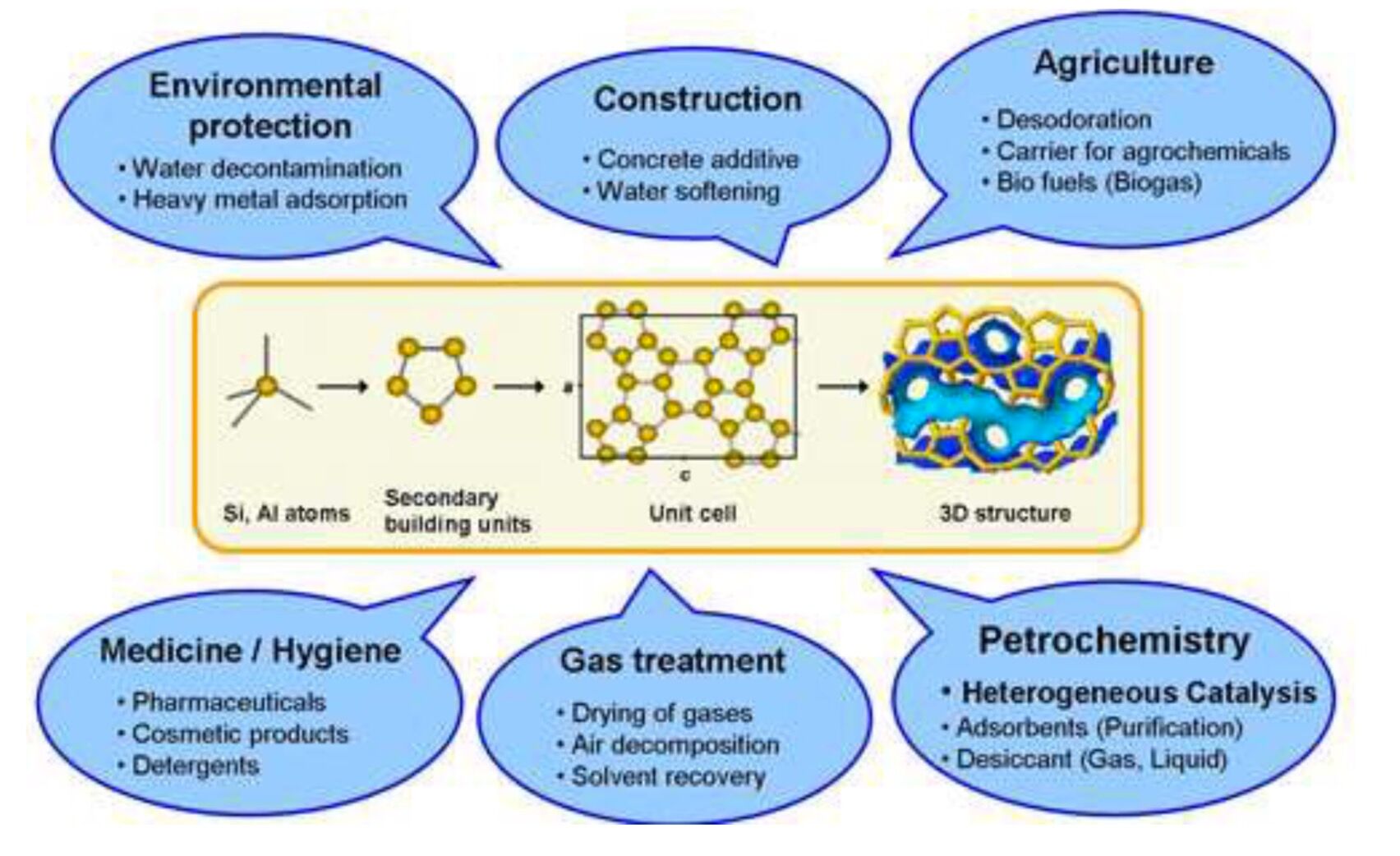
Does Zeolite Absorb Water . Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in.
from mybiohack.com
For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in.
The 19+ Benefits Of Zeolite And Clinoptilolites — MyBioHack Unlock
Does Zeolite Absorb Water Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range.
From ida-ore.com
Zeolite Stormwater Filtration Water Filtration with Zeolite Does Zeolite Absorb Water This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. For instance, zeolite 3a selectively adsorbs water from. Does Zeolite Absorb Water.
From netsolwater.com
What is the Importance of Zeolite water filtration medium Does Zeolite Absorb Water For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the. Does Zeolite Absorb Water.
From mybiohack.com
The 19+ Benefits Of Zeolite And Clinoptilolites — MyBioHack Unlock Does Zeolite Absorb Water For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. The zeolites trap the calcium and magnesium ions and release sodium ions in their place,. Does Zeolite Absorb Water.
From www.mdpi.com
Molecules Free FullText Influence of the Compensating Cation Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Zeolites are used as. Does Zeolite Absorb Water.
From pubs.acs.org
Zeolites in Adsorption Processes State of the Art and Future Prospects Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. For instance, zeolite 3a. Does Zeolite Absorb Water.
From www.youtube.com
7Zeolite Process II Softening of Hard Water1 YouTube Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. Water adsorption capacity, besides. Does Zeolite Absorb Water.
From seafood.media
Seafood Media Group Worldnews Benefits of Zeolite in Aquaculture Does Zeolite Absorb Water This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Zeolites are used as water softeners, to remove. Does Zeolite Absorb Water.
From www.youtube.com
Benefits of zeolite in fishpond.तालाब में गैस या अमोनिया हो रहा है तो Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. This review analyzes the current knowledge about the mechanistic aspects of. Does Zeolite Absorb Water.
From www.slideserve.com
PPT Pure Liquid Zeolite PowerPoint Presentation, free download ID Does Zeolite Absorb Water This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Zeolites. Does Zeolite Absorb Water.
From www.m-chemical.co.jp
Zeolite Membrane "ZEBREX™ Solvent Dehydration)" Products Does Zeolite Absorb Water Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. For instance, zeolite 3a selectively adsorbs water from. Does Zeolite Absorb Water.
From www.walmart.ca
Absorb Ammonia Zeolite,Water Absorb Ammonia Zeolite Absorb Ammonia Does Zeolite Absorb Water Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. This review analyzes the current knowledge about the. Does Zeolite Absorb Water.
From netsolwater.com
What is the se of Zeolite Water Treatment Media in WWTP Does Zeolite Absorb Water Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. Water. Does Zeolite Absorb Water.
From www.youtube.com
What Is Zeolite and How Can It Help the Detoxification Process? YouTube Does Zeolite Absorb Water Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water. Does Zeolite Absorb Water.
From www.naturallivingideas.com
6 Reasons You Should Place Zeolite Rocks Around Your Home Does Zeolite Absorb Water This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Zeolites are used as. Does Zeolite Absorb Water.
From www.walmart.com
Absorb Ammonia Zeolite Fish Absorb Ammonia Zeolite Water Absorb Ammonia Does Zeolite Absorb Water Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water. Does Zeolite Absorb Water.
From www.researchgate.net
Schematic representation of different types of zeolite membrane Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over. Does Zeolite Absorb Water.
From www.slideshare.net
Zeolite Process Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs. Does Zeolite Absorb Water.
From www.youtube.com
Ion Exchange Process Zeolite Process Demineralization Water Does Zeolite Absorb Water Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. For instance,. Does Zeolite Absorb Water.
From stock.adobe.com
The zeolite softening process is used for removing both the temporary Does Zeolite Absorb Water Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites.. Does Zeolite Absorb Water.
From www.researchgate.net
The preparation procedures of ZnSO 4 zeolite composite. [Colour figure Does Zeolite Absorb Water For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Water. Does Zeolite Absorb Water.
From www.grownmedz.co.za
Activated Zeolite Grownmedz Does Zeolite Absorb Water For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. The. Does Zeolite Absorb Water.
From www.researchgate.net
The Zeolite filtration unit Download Scientific Diagram Does Zeolite Absorb Water Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the. Does Zeolite Absorb Water.
From www.researchgate.net
Experimental process followed for the synthesis of zeolite from the Does Zeolite Absorb Water This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the. Does Zeolite Absorb Water.
From ou.edu
zeolites exhibit enhanced tolerance to waterThe Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water. Does Zeolite Absorb Water.
From pubs.acs.org
Adsorption Equilibria of Water Vapor on Zeolite 3A, Zeolite 13X, and Does Zeolite Absorb Water Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. For instance, zeolite 3a selectively adsorbs water. Does Zeolite Absorb Water.
From www.slideserve.com
PPT Pure Liquid Zeolite PowerPoint Presentation, free download ID Does Zeolite Absorb Water For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb). Does Zeolite Absorb Water.
From www.thesprucepets.com
When and How to Use Zeolite in Your Aquarium Does Zeolite Absorb Water Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. The zeolites trap the calcium and magnesium ions. Does Zeolite Absorb Water.
From www.youtube.com
Zeolite (Permutit) water Softening Method Engineering Chemistry IIT Does Zeolite Absorb Water Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. The. Does Zeolite Absorb Water.
From www.youtube.com
Zeolite process for water softening (Permutit process) Water Does Zeolite Absorb Water The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs. Does Zeolite Absorb Water.
From www.researchgate.net
Mechanism of Action of Zeolite Download Scientific Diagram Does Zeolite Absorb Water Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. Water. Does Zeolite Absorb Water.
From meanings.crystalsandjewelry.com
Zeolites Meanings, Properties and Powers The Complete Guide Does Zeolite Absorb Water This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. For instance, zeolite 3a selectively adsorbs water from. Does Zeolite Absorb Water.
From www.walmart.ca
Fish Tank Absorb Ammonia Zeolite,Water Absorb Ammonia Zeolite Fish Tank Does Zeolite Absorb Water Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. The zeolites trap the calcium and magnesium ions. Does Zeolite Absorb Water.
From netsolwater.com
What makes water treatment with zeolite media a good filter Does Zeolite Absorb Water This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Zeolites are used as water softeners, to remove calcium ions, which react with soap to form scum. For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range.. Does Zeolite Absorb Water.
From www.youtube.com
Adsorption on a Zeolite (Interactive) YouTube Does Zeolite Absorb Water For instance, zeolite 3a selectively adsorbs water from gas streams and liquid solvents, and zeolite 5a selectively adsorbs linear over branched hydrocarbons in the gasoline range. This review analyzes the current knowledge about the mechanistic aspects of water adsorption and nucleation on zeolites. Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these. Does Zeolite Absorb Water.
From www.explainthatstuff.com
What are zeolites? How do zeolite catalysts work? Does Zeolite Absorb Water Water adsorption capacity, besides hydrophilicity, is the most important parameter for the performance of zeolites in these devices as. Water (h 2 o) molecules within microporous environments form intricate structures through hydrogen bonds (hb) that. The zeolites trap the calcium and magnesium ions and release sodium ions in their place, so the water becomes softer but richer in. For instance,. Does Zeolite Absorb Water.