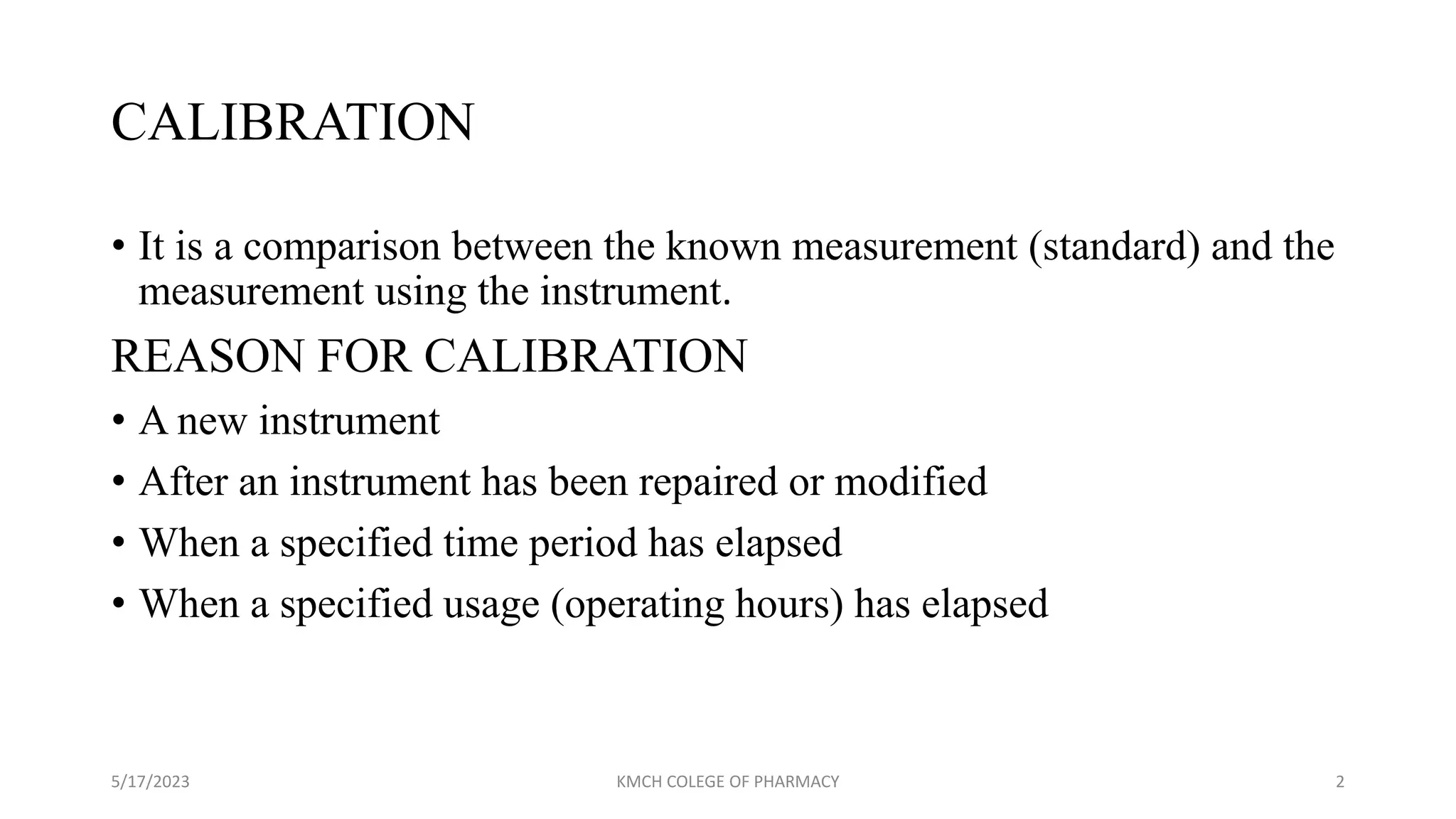
Usfda Guidelines For Calibration And Validation . § 58.63 maintenance and calibration of equipment. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. (a) equipment shall be adequately inspected, cleaned, and maintained. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process.
from www.slideshare.net
Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. (a) equipment shall be adequately inspected, cleaned, and maintained. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. § 58.63 maintenance and calibration of equipment. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process.
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx
Usfda Guidelines For Calibration And Validation Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. § 58.63 maintenance and calibration of equipment. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. (a) equipment shall be adequately inspected, cleaned, and maintained.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation (a) equipment shall be adequately inspected, cleaned, and maintained. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Guidelines for the validation of analytical methods for the detection of microbial pathogens. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. § 58.63 maintenance and calibration of equipment. Validation studies • define the test methodology that will be used for the process, may. Usfda Guidelines For Calibration And Validation.
From www.scribd.com
Pharmaceutical Guidelines_ISO, FDA, USFDA, ICH, WHO, GMP, MHRA Usfda Guidelines For Calibration And Validation It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. (a) equipment shall be adequately inspected,. Usfda Guidelines For Calibration And Validation.
From calibrationawareness.com
How to Differentiate Calibration, Verification, and Validation Usfda Guidelines For Calibration And Validation The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. § 58.63 maintenance and calibration of equipment. The guideline is directed to common uses of analytical procedures, such as. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
DATION OF EQUIPMENT ICH AND WHO GUIDELINES FOR CALIBRATION AND VALIDA… Usfda Guidelines For Calibration And Validation Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. § 58.63 maintenance and calibration of equipment. This guidance outlines the general principles and approaches that fda considers appropriate elements of process. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
USFDA guidelines for bioanalytical method validation Usfda Guidelines For Calibration And Validation It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. (a) equipment shall be adequately inspected, cleaned, and maintained. § 58.63 maintenance and calibration of equipment. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. The guideline is directed to common uses of analytical procedures, such as assay,. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
USFDA guidelines for bioanalytical method validation PPT Usfda Guidelines For Calibration And Validation § 58.63 maintenance and calibration of equipment. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and.. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
3.ICH &WHO Guidelines for Calibration And Validation of Equipments.pptx Usfda Guidelines For Calibration And Validation This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. § 58.63 maintenance and calibration of equipment. (a) equipment shall be adequately inspected, cleaned, and maintained. It discusses validation characteristics like accuracy,. Usfda Guidelines For Calibration And Validation.
From www.researchgate.net
(PDF) ICH and WHO Guideline for validation and calibration Usfda Guidelines For Calibration And Validation Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. (a) equipment shall be adequately inspected, cleaned, and maintained. Guidelines for the validation of analytical methods for the detection. Usfda Guidelines For Calibration And Validation.
From www.researchgate.net
(PDF) USFDA Guidelines on Process Validation A Review Usfda Guidelines For Calibration And Validation Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. § 58.63 maintenance and calibration of equipment. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation. Usfda Guidelines For Calibration And Validation.
From www.youtube.com
Quality Metrics Data Submission USFDA Guidance USFDA Guidelines Usfda Guidelines For Calibration And Validation Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. The guideline is directed to. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
USFDA guidelines for bioanalytical method validation Usfda Guidelines For Calibration And Validation This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. § 58.63 maintenance and calibration of equipment. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. The concept of validation has changed radically since the publication of the fd a's revised. Usfda Guidelines For Calibration And Validation.
From www.scribd.com
USFDA Food And Drug Administration Verification And Validation Usfda Guidelines For Calibration And Validation Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. § 58.63 maintenance and calibration of equipment. (a) equipment shall be adequately inspected, cleaned, and maintained. Validation studies • define the test methodology that will be used for the process,. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation (a) equipment shall be adequately inspected, cleaned, and maintained. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. § 58.63 maintenance and calibration of equipment. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Guidelines for the validation of analytical methods for the detection of. Usfda Guidelines For Calibration And Validation.
From www.studypool.com
SOLUTION Who guidelines for calibration and validation of equipments Usfda Guidelines For Calibration And Validation The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. (a) equipment shall be adequately inspected, cleaned, and maintained. This guidance outlines the general principles and approaches that fda. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. (a) equipment shall be adequately inspected, cleaned, and maintained. § 58.63 maintenance and calibration of equipment. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. Validation studies • define the test. Usfda Guidelines For Calibration And Validation.
From www.studypool.com
SOLUTION Who guidelines for calibration and validation of equipments Usfda Guidelines For Calibration And Validation Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. The guideline. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation (a) equipment shall be adequately inspected, cleaned, and maintained. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. §. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. This guidance outlines the general principles and approaches that. Usfda Guidelines For Calibration And Validation.
From www.laafon.com
USFDA inspection checklist for pharmaceutical facility Usfda Guidelines For Calibration And Validation (a) equipment shall be adequately inspected, cleaned, and maintained. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. § 58.63 maintenance and calibration of equipment. The concept of validation. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
USFDA guidelines for bioanalytical method validation PPT Usfda Guidelines For Calibration And Validation The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. (a) equipment shall be adequately inspected, cleaned, and maintained. Guidelines for the validation of analytical methods for the detection of microbial pathogens. Usfda Guidelines For Calibration And Validation.
From dokumen.tips
(PDF) Guidelines for calibration DOKUMEN.TIPS Usfda Guidelines For Calibration And Validation Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. (a) equipment shall be adequately inspected, cleaned, and maintained. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. §. Usfda Guidelines For Calibration And Validation.
From www.pharmaspecialists.com
Difference Between Calibration and Validation Usfda Guidelines For Calibration And Validation § 58.63 maintenance and calibration of equipment. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation. Usfda Guidelines For Calibration And Validation.
From www.slideserve.com
PPT Evaluating Change in Hazard in Clinical Trials With TimetoEvent Usfda Guidelines For Calibration And Validation § 58.63 maintenance and calibration of equipment. (a) equipment shall be adequately inspected, cleaned, and maintained. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. The guideline is directed to common uses of analytical procedures, such. Usfda Guidelines For Calibration And Validation.
From www.scribd.com
Checklist For USFDA Audit Preparation in Quality Control Usfda Guidelines For Calibration And Validation § 58.63 maintenance and calibration of equipment. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit. Usfda Guidelines For Calibration And Validation.
From www.indiamart.com
Offline USFDA Certified Calibration Management Solution, Free Download Usfda Guidelines For Calibration And Validation Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. § 58.63 maintenance and calibration of equipment. The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. (a) equipment shall be adequately inspected, cleaned, and maintained. Validation studies • define the test. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
Ich & who guidelines for calibration and validation 22 PPT Usfda Guidelines For Calibration And Validation It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. § 58.63 maintenance and calibration of equipment. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. (a) equipment shall be adequately. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation (a) equipment shall be adequately inspected, cleaned, and maintained. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. The. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
ICH USFDA Validation Differences Usfda Guidelines For Calibration And Validation This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. (a) equipment shall be adequately inspected, cleaned, and maintained. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. § 58.63 maintenance and calibration of equipment. Validation studies • define the test methodology that will. Usfda Guidelines For Calibration And Validation.
From www.pharmaspecialists.com
Calibration As per EU and USFDA GMP Requirements Usfda Guidelines For Calibration And Validation § 58.63 maintenance and calibration of equipment. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. The concept of. Usfda Guidelines For Calibration And Validation.
From www.youtube.com
USFDA Guidance for Pharmaceutical Quality System USFDA Guidelines for Usfda Guidelines For Calibration And Validation The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. § 58.63 maintenance and calibration of equipment. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. The concept of validation has changed radically since the publication of the fd a's revised guidance. Usfda Guidelines For Calibration And Validation.
From dokumen.tips
(PDF) Usfda guidelines DOKUMEN.TIPS Usfda Guidelines For Calibration And Validation The concept of validation has changed radically since the publication of the fd a's revised guidance for industr y on process. (a) equipment shall be adequately inspected, cleaned, and maintained. This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. Guidelines for the validation of analytical methods for the detection of microbial. Usfda Guidelines For Calibration And Validation.
From swarali-bhakre.medium.com
USFDA Guidelines for Equipment Qualification by IZiel Healthcare Medium Usfda Guidelines For Calibration And Validation It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. (a) equipment shall be adequately inspected, cleaned, and maintained. Validation studies • define the test methodology that will be used for the process, may be technology & decontamination process. § 58.63 maintenance and calibration of equipment. The guideline is directed to common uses of analytical procedures, such. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
CALIBRATION PROTOCOL AS PER USFDA GUIDELINES.pptx Usfda Guidelines For Calibration And Validation This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the. (a) equipment shall be adequately inspected, cleaned, and maintained. § 58.63 maintenance and calibration of equipment. It discusses validation characteristics like accuracy, precision, specificity, linearity, range, detection limit and. Guidelines for the validation of analytical methods for the detection of microbial pathogens. Usfda Guidelines For Calibration And Validation.
From www.slideshare.net
USFDA guidelines for bioanalytical method validation PPT Usfda Guidelines For Calibration And Validation § 58.63 maintenance and calibration of equipment. The guideline is directed to common uses of analytical procedures, such as assay, potency, purity, impurity (quantitative or limit test),. Guidelines for the validation of analytical methods for the detection of microbial pathogens in foods and feeds. The concept of validation has changed radically since the publication of the fd a's revised guidance. Usfda Guidelines For Calibration And Validation.