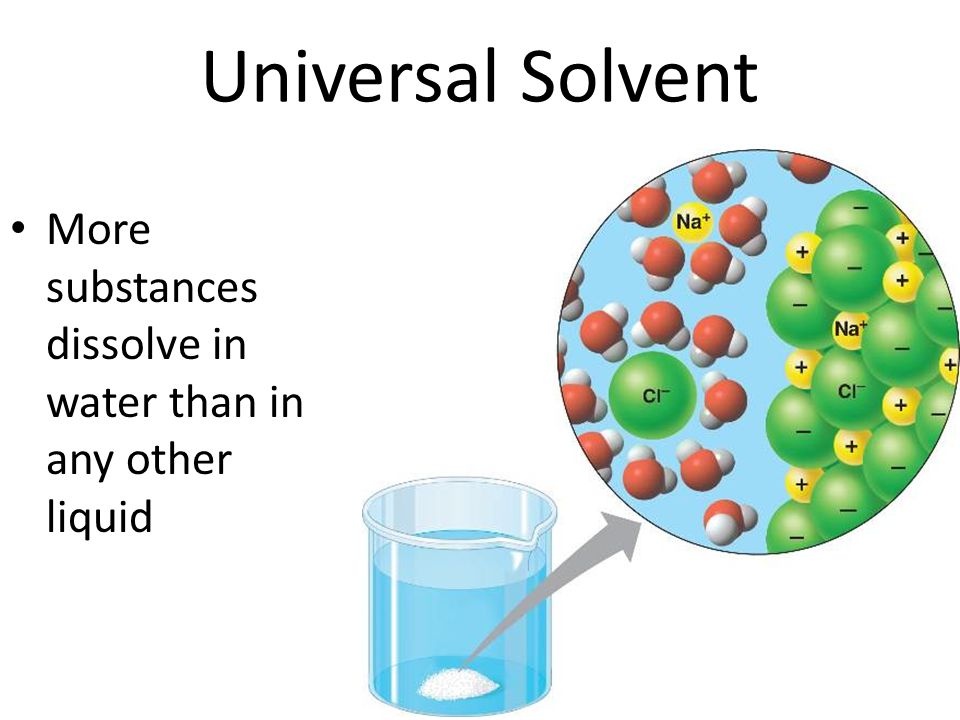
Universal Solvent . Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Find out what factors affect water's solubility and what compounds are not soluble in water.
from app.emaze.com
Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Find out what factors affect water's solubility and what compounds are not soluble in water.
water on emaze
Universal Solvent Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical.
From www.gocopy.com.au
MTN PRO Solvent 400ml Spray Universal Solvent GoCopy Universal Solvent Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. The term 'universal solvent' refers to water's unique ability to dissolve. Universal Solvent.
From
Universal Solvent Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn how water's polar molecules and ionic bonds make it. Universal Solvent.
From www.thoughtco.com
Why Is Water the Universal Solvent? Universal Solvent Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Find. Universal Solvent.
From
Universal Solvent Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. Find out what factors affect water's solubility and what compounds are not soluble in water. A universal solvent is a substance that. Universal Solvent.
From studylib.net
Water The Universal Solvent! Powerpoint Universal Solvent Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn how water's polarity and ionic bonds make. Universal Solvent.
From medium.com
Water The Universal Solvent BICPUC Medium Universal Solvent Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. Learn why water is called the. Universal Solvent.
From
Universal Solvent Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. The term. Universal Solvent.
From
Universal Solvent Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn how water's polar. Universal Solvent.
From
Universal Solvent Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Find out. Universal Solvent.
From brainly.com
Why is water called universal solvent? Universal Solvent Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for.. Universal Solvent.
From www.slideserve.com
PPT Properties of Solutions PowerPoint Presentation, free download Universal Solvent Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn why water. Universal Solvent.
From
Universal Solvent Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn. Universal Solvent.
From
Universal Solvent Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Find out what factors affect water's solubility and. Universal Solvent.
From
Universal Solvent The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn how water's polar. Universal Solvent.
From
Universal Solvent Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving.. Universal Solvent.
From imbros.com.au
The universal solvent a guide to water purification and deionisation Universal Solvent Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn. Universal Solvent.
From
Universal Solvent The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn why water is the universal solvent. Universal Solvent.
From
Universal Solvent Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. A universal solvent. Universal Solvent.
From
Universal Solvent Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving.. Universal Solvent.
From
Universal Solvent Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. The term 'universal solvent' refers to water's unique. Universal Solvent.
From
Universal Solvent The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. Learn why water is the. Universal Solvent.
From
Universal Solvent Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. The term. Universal Solvent.
From
Universal Solvent Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Find out what factors affect water's solubility and what compounds are not soluble in. Universal Solvent.
From
Universal Solvent The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of. Universal Solvent.
From www.thoughtco.com
Universal Solvent Definition Universal Solvent Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Learn. Universal Solvent.
From
Universal Solvent Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents.. Universal Solvent.
From powersuppliesgroupltd.co.uk
Universal Solvent Cleaner /Degreaser HP Type Universal Solvent Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn how water's. Universal Solvent.
From
Universal Solvent Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn how water's. Universal Solvent.
From
Universal Solvent Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Find out what factors affect. Universal Solvent.
From
Universal Solvent Learn why water is called the universal solvent, how alkahest was a hypothetical universal solvent, and what are the types and uses of solvents. Learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density affect its dissolving. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn. Universal Solvent.
From
Universal Solvent Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn why water is called the universal solvent and how it. Universal Solvent.
From
Universal Solvent Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polarity and ionic bonds make it dissolve more substances than any other chemical. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn why water is the universal solvent and how its polarity, surface tension, specific. Universal Solvent.
From www.youtube.com
Science, Grade 9 Water Polarity & Universal Solvent Lido Learning Universal Solvent Find out what factors affect water's solubility and what compounds are not soluble in water. The term 'universal solvent' refers to water's unique ability to dissolve a wide variety of substances, making it an essential medium for. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. Learn why water is the. Universal Solvent.
From en.pinturaslepanto.com
Universal solvent Pinturas Lepanto Paint manufacturer for Universal Solvent Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's polar molecules and ionic bonds make it a great solvent for many substances, including salt. A universal solvent is a substance that dissolves most chemicals, but no such solvent exists. Learn why water is the universal solvent and how its polarity, surface. Universal Solvent.
From
Universal Solvent Find out what factors affect water's solubility and what compounds are not soluble in water. Learn how water's unique properties and polarity make it a universal solvent that can dissolve or dissociate many substances. Learn why water is called the universal solvent and how it dissolves more substances than any other liquid. Learn how water's polarity and ionic bonds make. Universal Solvent.