Zinc Iodide Solubility In Methanol . The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Solubility of alcohols in water. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids.
from www.chegg.com
In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Solubility of alcohols in water.
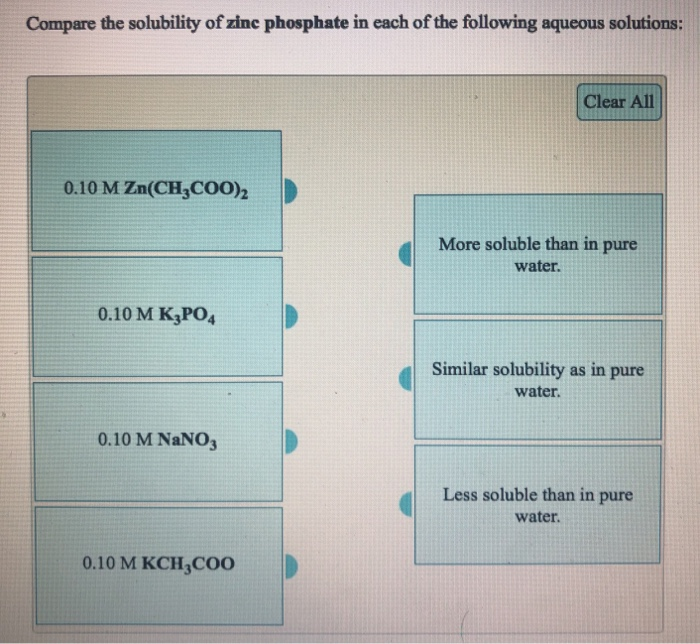
Solved Compare the solubility of zinc sulfide in each of the
Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Solubility of alcohols in water. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile.
From www.chegg.com
Solved Suppose 0.195 g of zinc iodide is dissolved in 100. Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Compare the solubility of lead iodide in each of the Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Solubility of alcohols in water. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. In this section we will. Zinc Iodide Solubility In Methanol.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Solubility In Methanol Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. In this section we will apply chemical equilibria to the concept of solubility and introduce. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Solubility In Methanol Solubility of alcohols in water. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the. Zinc Iodide Solubility In Methanol.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation Zinc Iodide Solubility In Methanol The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Solubility of alcohols in water. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the. Zinc Iodide Solubility In Methanol.
From www.dexform.com
Solubility Rules Chart in Word and Pdf formats Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Solubility of alcohols in water. In this section we will. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Solubility of alcohols in water. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide. Zinc Iodide Solubility In Methanol.
From www.youtube.com
Finding the Empirical Formula For Zinc Iodide General Chemistry Zinc Iodide Solubility In Methanol The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Alcohols and water have the ability to form hydrogen. Zinc Iodide Solubility In Methanol.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Iodide Solubility In Methanol In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Solubility of alcohols in water. The stability of iodozinc complexes in methanol is medium strong as the zinc and. Zinc Iodide Solubility In Methanol.
From www.researchgate.net
The solubility curve of FBMS in the mixture of methanol and MTBE with Zinc Iodide Solubility In Methanol 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. In this section we will apply chemical equilibria to the. Zinc Iodide Solubility In Methanol.
From www.pinterest.com
Solubility Rules Chart for Chemistry Classroom 11th chemistry Zinc Iodide Solubility In Methanol In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more. Zinc Iodide Solubility In Methanol.
From pubs.acs.org
On the Mechanism of the Conversion of Methanol to 2,2,3Trimethylbutane Zinc Iodide Solubility In Methanol In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two. Zinc Iodide Solubility In Methanol.
From studylib.net
Determination of a chemical formula the synthesis of zinc iodide Zinc Iodide Solubility In Methanol The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the ability to form hydrogen bonds. Zinc Iodide Solubility In Methanol.
From www.researchgate.net
(PDF) Structure of Methanol Solvated Iodozinc(II) Complexes in Solution Zinc Iodide Solubility In Methanol 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide. Zinc Iodide Solubility In Methanol.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation Zinc Iodide Solubility In Methanol The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Solubility of alcohols in water. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the. Zinc Iodide Solubility In Methanol.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Solubility of alcohols in water. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. In this section we will apply chemical equilibria to the. Zinc Iodide Solubility In Methanol.
From www.nagwa.com
Question Video Identifying the Reason for Melting Zinc Iodide before Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. 133 rows although all compounds have a characteristic solubility in. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Suppose 3.77 g of zinc iodide is dissolved in 350.mL Zinc Iodide Solubility In Methanol Solubility of alcohols in water. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Solubility In Methanol 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. In this section we will apply chemical equilibria to the. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved the do we need to dissolve the iodine in methanol? Zinc Iodide Solubility In Methanol Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds. Zinc Iodide Solubility In Methanol.
From www.numerade.com
SOLVED 34. On the basis of the general solubility rules given in Table Zinc Iodide Solubility In Methanol 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide. Zinc Iodide Solubility In Methanol.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation Zinc Iodide Solubility In Methanol Solubility of alcohols in water. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved Suppose 1.22 g of zinc iodide is dissolved in 350.mL Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Solubility of alcohols in water. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide. Zinc Iodide Solubility In Methanol.
From mavink.com
Metal Solubility Chart Zinc Iodide Solubility In Methanol In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more. Zinc Iodide Solubility In Methanol.
From www.youtube.com
How to Write the Formula for Zinc iodide YouTube Zinc Iodide Solubility In Methanol The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will apply chemical equilibria to the. Zinc Iodide Solubility In Methanol.
From www.chegg.com
Solved the do we need to dissolve the iodine in methanol? Zinc Iodide Solubility In Methanol The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will. Zinc Iodide Solubility In Methanol.
From www.pw.live
Zinc Iodide Formula, Structure, Properties And Uses Zinc Iodide Solubility In Methanol Solubility of alcohols in water. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will apply chemical equilibria to the concept of solubility and introduce. Zinc Iodide Solubility In Methanol.
From www.transtutors.com
(Get Answer) Finding The Empirical Formula Of Zinc Iodide Report Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Solubility of alcohols in water. The stability of iodozinc complexes in methanol is medium strong as the zinc and. Zinc Iodide Solubility In Methanol.
From nextgenpetstudent.activatelearning.com
NextGenPET Student Resources Zinc Iodide Solubility In Methanol 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. Alcohols and water have the ability to form hydrogen bonds. Zinc Iodide Solubility In Methanol.
From www.researchgate.net
Depiction of major Raman modes of zinc iodide and zinc chloride species Zinc Iodide Solubility In Methanol Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more weakly solvated than in water but more strongly than in acetonitrile. In this section we will. Zinc Iodide Solubility In Methanol.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Iodide Solubility In Methanol In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The stability of iodozinc complexes in methanol is medium strong as the zinc and iodide ions are both more. Zinc Iodide Solubility In Methanol.
From keplarllp.com
😍 What is the formula for zinc iodide. Formula for zinc iodide. 20190204 Zinc Iodide Solubility In Methanol Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant,. Zinc Iodide Solubility In Methanol.