Single Aluminum Atom In Grams . Find the molar mass of the substance in grams per mole (g/mol). The model mass of a single aluminum atom is 26.98g promote. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. First we find the atomic mass of al. Dividing 26.98 6.02 ×1023 yields a mass of. One more piece of aluminum.
from mavink.com
How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. Find the molar mass of the substance in grams per mole (g/mol). One more piece of aluminum. First we find the atomic mass of al. The model mass of a single aluminum atom is 26.98g promote. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Dividing 26.98 6.02 ×1023 yields a mass of.
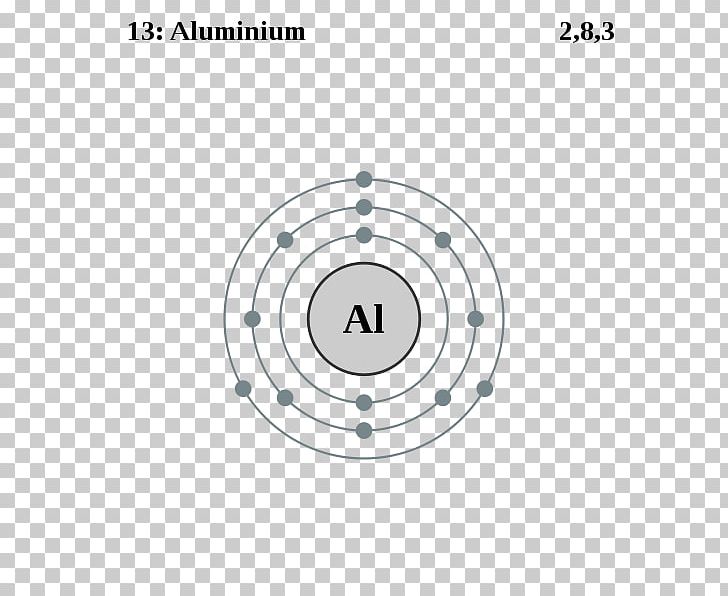
Aluminum Shell Diagram
Single Aluminum Atom In Grams Find the molar mass of the substance in grams per mole (g/mol). Dividing 26.98 6.02 ×1023 yields a mass of. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. One more piece of aluminum. The model mass of a single aluminum atom is 26.98g promote. There are two steps to find the mass of the aluminum (al) atom. First we find the atomic mass of al. Find the molar mass of the substance in grams per mole (g/mol). How to convert atom to gram?
From www.alamy.com
A Aluminium atom diagram illustration Stock Vector Image & Art Alamy Single Aluminum Atom In Grams How to convert atom to gram? Find the molar mass of the substance in grams per mole (g/mol). Dividing 26.98 6.02 ×1023 yields a mass of. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. First we find the atomic mass of al. The model mass of a single aluminum atom is 26.98g. Single Aluminum Atom In Grams.
From www.shutterstock.com
Diagram Aluminum Atom Periodic Table Element Stock Vector (Royalty Free Single Aluminum Atom In Grams First we find the atomic mass of al. One more piece of aluminum. Find the molar mass of the substance in grams per mole (g/mol). How to convert atom to gram? Dividing 26.98 6.02 ×1023 yields a mass of. There are two steps to find the mass of the aluminum (al) atom. The model mass of a single aluminum atom. Single Aluminum Atom In Grams.
From www.dreamstime.com
Aluminium Atom on a White Background Stock Illustration Illustration Single Aluminum Atom In Grams Dividing 26.98 6.02 ×1023 yields a mass of. There are two steps to find the mass of the aluminum (al) atom. First we find the atomic mass of al. One more piece of aluminum. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Find the molar mass of the substance in grams per. Single Aluminum Atom In Grams.
From proper-cooking.info
Aluminum Atomic Structure Single Aluminum Atom In Grams Dividing 26.98 6.02 ×1023 yields a mass of. There are two steps to find the mass of the aluminum (al) atom. Find the molar mass of the substance in grams per mole (g/mol). The model mass of a single aluminum atom is 26.98g promote. First we find the atomic mass of al. One mole of aluminum atoms has a mass. Single Aluminum Atom In Grams.
From www.enfo.hu
Aluminium ENvironmental inFOrmation Single Aluminum Atom In Grams The model mass of a single aluminum atom is 26.98g promote. There are two steps to find the mass of the aluminum (al) atom. Find the molar mass of the substance in grams per mole (g/mol). One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Dividing 26.98 6.02 ×1023 yields a mass of.. Single Aluminum Atom In Grams.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Single Aluminum Atom In Grams Dividing 26.98 6.02 ×1023 yields a mass of. There are two steps to find the mass of the aluminum (al) atom. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Find the molar mass of the substance in grams per mole (g/mol). One more piece of aluminum. First we find the atomic mass. Single Aluminum Atom In Grams.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Single Aluminum Atom In Grams One more piece of aluminum. There are two steps to find the mass of the aluminum (al) atom. The model mass of a single aluminum atom is 26.98g promote. How to convert atom to gram? Dividing 26.98 6.02 ×1023 yields a mass of. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Find. Single Aluminum Atom In Grams.
From fr.dreamstime.com
Modèle De L'atome D'aluminium Illustration de Vecteur Illustration du Single Aluminum Atom In Grams One more piece of aluminum. How to convert atom to gram? Find the molar mass of the substance in grams per mole (g/mol). The model mass of a single aluminum atom is 26.98g promote. There are two steps to find the mass of the aluminum (al) atom. One mole of aluminum atoms has a mass of 26.98 grams, and contains. Single Aluminum Atom In Grams.
From h-o-m-e.org
Avogadro's Number The Key to Converting Grams to Atoms Single Aluminum Atom In Grams Dividing 26.98 6.02 ×1023 yields a mass of. How to convert atom to gram? One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Find the molar mass of the substance in grams per mole (g/mol). There are two steps to find the mass of the aluminum (al) atom. One more piece of aluminum.. Single Aluminum Atom In Grams.
From www.slideserve.com
PPT Molecular Weight and Atomic Weight PowerPoint Presentation, free Single Aluminum Atom In Grams How to convert atom to gram? One more piece of aluminum. Find the molar mass of the substance in grams per mole (g/mol). One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. There are two steps to find the mass of the aluminum (al) atom. First we find the atomic mass of al.. Single Aluminum Atom In Grams.
From steamever.weebly.com
Conversion Of Atoms To Grams steamever Single Aluminum Atom In Grams Find the molar mass of the substance in grams per mole (g/mol). The model mass of a single aluminum atom is 26.98g promote. How to convert atom to gram? Dividing 26.98 6.02 ×1023 yields a mass of. There are two steps to find the mass of the aluminum (al) atom. One more piece of aluminum. One mole of aluminum atoms. Single Aluminum Atom In Grams.
From www.coursehero.com
[Solved] what is the weight of one aluminum atom in grams? Course Hero Single Aluminum Atom In Grams First we find the atomic mass of al. Find the molar mass of the substance in grams per mole (g/mol). One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. There are two steps to find the mass of the aluminum (al) atom. How to convert atom to gram? One more piece of aluminum.. Single Aluminum Atom In Grams.
From www.dreamstime.com
A Aluminium atom diagram stock vector. Illustration of vector 142735160 Single Aluminum Atom In Grams How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. Dividing 26.98 6.02 ×1023 yields a mass of. One more piece of aluminum. Find the molar mass of the substance in grams per mole (g/mol). The model mass of a single aluminum atom is 26.98g promote. First we find the atomic. Single Aluminum Atom In Grams.
From www.youtube.com
grams to atoms calculations YouTube Single Aluminum Atom In Grams The model mass of a single aluminum atom is 26.98g promote. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. How to convert atom to gram? Dividing 26.98 6.02 ×1023 yields a mass of. There are two steps to find the mass of the aluminum (al) atom. Find the molar mass of the. Single Aluminum Atom In Grams.
From www.kindpng.com
Aluminum Periodic Table Atom, HD Png Download kindpng Single Aluminum Atom In Grams The model mass of a single aluminum atom is 26.98g promote. Dividing 26.98 6.02 ×1023 yields a mass of. There are two steps to find the mass of the aluminum (al) atom. Find the molar mass of the substance in grams per mole (g/mol). First we find the atomic mass of al. One mole of aluminum atoms has a mass. Single Aluminum Atom In Grams.
From ar.inspiredpencil.com
Aluminum Bohr Model Single Aluminum Atom In Grams How to convert atom to gram? One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Find the molar mass of the substance in grams per mole (g/mol). There are two steps to find the mass of the aluminum (al) atom. First we find the atomic mass of al. The model mass of a. Single Aluminum Atom In Grams.
From periodictable.me
How Can We Find Electron Configuration For Aluminium (Al) Single Aluminum Atom In Grams Dividing 26.98 6.02 ×1023 yields a mass of. First we find the atomic mass of al. How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. The model mass of a single aluminum atom is. Single Aluminum Atom In Grams.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Single Aluminum Atom In Grams One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. The model mass of a single aluminum atom is 26.98g promote. One more piece of aluminum. Dividing 26.98 6.02 ×1023 yields a mass of. First. Single Aluminum Atom In Grams.
From mavink.com
Aluminum Shell Diagram Single Aluminum Atom In Grams First we find the atomic mass of al. One more piece of aluminum. There are two steps to find the mass of the aluminum (al) atom. The model mass of a single aluminum atom is 26.98g promote. Dividing 26.98 6.02 ×1023 yields a mass of. How to convert atom to gram? Find the molar mass of the substance in grams. Single Aluminum Atom In Grams.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Single Aluminum Atom In Grams One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Dividing 26.98 6.02 ×1023 yields a mass of. How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. Find the molar mass of the substance in grams per mole (g/mol). The model mass of a. Single Aluminum Atom In Grams.
From www.alamy.com
Aluminium periodic element chemical symbol. Aluminum atom element Single Aluminum Atom In Grams Dividing 26.98 6.02 ×1023 yields a mass of. The model mass of a single aluminum atom is 26.98g promote. First we find the atomic mass of al. How to convert atom to gram? One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. One more piece of aluminum. Find the molar mass of the. Single Aluminum Atom In Grams.
From www.alamy.com
Al Aluminum Chemical Element Periodic Table. Single element vector Single Aluminum Atom In Grams One more piece of aluminum. How to convert atom to gram? First we find the atomic mass of al. There are two steps to find the mass of the aluminum (al) atom. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. The model mass of a single aluminum atom is 26.98g promote. Dividing. Single Aluminum Atom In Grams.
From www.alamy.com
Al Aluminium Chemical Element Periodic Table. Single vector Single Aluminum Atom In Grams One more piece of aluminum. Find the molar mass of the substance in grams per mole (g/mol). How to convert atom to gram? The model mass of a single aluminum atom is 26.98g promote. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. First we find the atomic mass of al. There are. Single Aluminum Atom In Grams.
From www.sciencephoto.com
Aluminium, atomic structure Stock Image C013/1526 Science Photo Single Aluminum Atom In Grams How to convert atom to gram? One more piece of aluminum. Find the molar mass of the substance in grams per mole (g/mol). There are two steps to find the mass of the aluminum (al) atom. First we find the atomic mass of al. The model mass of a single aluminum atom is 26.98g promote. Dividing 26.98 6.02 ×1023 yields. Single Aluminum Atom In Grams.
From slideplayer.com
Introduction to Material Science ppt download Single Aluminum Atom In Grams How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. The model mass of a single aluminum atom is 26.98g promote. First we find the atomic mass of al. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. One more piece of aluminum. Dividing. Single Aluminum Atom In Grams.
From www.sciencephoto.com
Aluminium, atomic structure Stock Image C023/2476 Science Photo Single Aluminum Atom In Grams The model mass of a single aluminum atom is 26.98g promote. One more piece of aluminum. There are two steps to find the mass of the aluminum (al) atom. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Find the molar mass of the substance in grams per mole (g/mol). First we find. Single Aluminum Atom In Grams.
From theeducationinfo.com
Grams to atoms calculator step by step guide Single Aluminum Atom In Grams How to convert atom to gram? One more piece of aluminum. Find the molar mass of the substance in grams per mole (g/mol). The model mass of a single aluminum atom is 26.98g promote. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. First we find the atomic mass of al. There are. Single Aluminum Atom In Grams.
From mavink.com
Aluminum Atom Model Single Aluminum Atom In Grams One more piece of aluminum. How to convert atom to gram? One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. There are two steps to find the mass of the aluminum (al) atom. The model mass of a single aluminum atom is 26.98g promote. Find the molar mass of the substance in grams. Single Aluminum Atom In Grams.
From cabinet.matttroy.net
Aluminum Periodic Table Protons Neutrons And Electrons Matttroy Single Aluminum Atom In Grams Find the molar mass of the substance in grams per mole (g/mol). One more piece of aluminum. How to convert atom to gram? First we find the atomic mass of al. The model mass of a single aluminum atom is 26.98g promote. Dividing 26.98 6.02 ×1023 yields a mass of. One mole of aluminum atoms has a mass of 26.98. Single Aluminum Atom In Grams.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Al Aluminium Element Information Facts, Properties, Trends, Uses and Single Aluminum Atom In Grams Dividing 26.98 6.02 ×1023 yields a mass of. One more piece of aluminum. There are two steps to find the mass of the aluminum (al) atom. How to convert atom to gram? Find the molar mass of the substance in grams per mole (g/mol). One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms.. Single Aluminum Atom In Grams.
From www.vectorstock.com
Diagram representation of the element aluminium Vector Image Single Aluminum Atom In Grams The model mass of a single aluminum atom is 26.98g promote. Dividing 26.98 6.02 ×1023 yields a mass of. How to convert atom to gram? There are two steps to find the mass of the aluminum (al) atom. One more piece of aluminum. First we find the atomic mass of al. One mole of aluminum atoms has a mass of. Single Aluminum Atom In Grams.
From mavink.com
Aluminum Shell Diagram Single Aluminum Atom In Grams How to convert atom to gram? One more piece of aluminum. The model mass of a single aluminum atom is 26.98g promote. Find the molar mass of the substance in grams per mole (g/mol). There are two steps to find the mass of the aluminum (al) atom. First we find the atomic mass of al. Dividing 26.98 6.02 ×1023 yields. Single Aluminum Atom In Grams.
From www.sciencephoto.com
Aluminium, atomic structure Stock Image C019/7644 Science Photo Single Aluminum Atom In Grams One more piece of aluminum. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Dividing 26.98 6.02 ×1023 yields a mass of. First we find the atomic mass of al. Find the molar mass of the substance in grams per mole (g/mol). How to convert atom to gram? There are two steps to. Single Aluminum Atom In Grams.
From www.youtube.com
How to Find the Mass of One Atom of Aluminum (Al) YouTube Single Aluminum Atom In Grams The model mass of a single aluminum atom is 26.98g promote. How to convert atom to gram? First we find the atomic mass of al. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. Dividing 26.98 6.02 ×1023 yields a mass of. One more piece of aluminum. Find the molar mass of the. Single Aluminum Atom In Grams.
From www.chegg.com
Solved What is the average mass of a single aluminum atom in Single Aluminum Atom In Grams There are two steps to find the mass of the aluminum (al) atom. The model mass of a single aluminum atom is 26.98g promote. One mole of aluminum atoms has a mass of 26.98 grams, and contains 6.02× 1023 atoms. How to convert atom to gram? First we find the atomic mass of al. Find the molar mass of the. Single Aluminum Atom In Grams.