What Is Electron Configuration For Iron . It is termed as ferromagnetic materials. Its valence orbitals are the 4s and 3d 's. Writing the electron configuration, you really only need the. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. In order to write the electron configuration for iron (fe) we first need to know the. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Iron is a transition metal with the atomic. Electronic configuration of iron is [ar] 3d64s2. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. The electron configuration of iron (fe) is [ar] 3d6 4s2. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron has 26 electrons in total.
from electronschematic.blogspot.com
Its valence orbitals are the 4s and 3d 's. The electron configuration of iron (fe) is [ar] 3d6 4s2. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. It is termed as ferromagnetic materials. Electronic configuration of iron is [ar] 3d64s2. Writing the electron configuration, you really only need the. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron is a transition metal with the atomic. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. In order to write the electron configuration for iron (fe) we first need to know the.
Electron Schematic What Is The Longhand Electron Configuration For Iron
What Is Electron Configuration For Iron Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. The electron configuration of iron (fe) is [ar] 3d6 4s2. It is termed as ferromagnetic materials. Electronic configuration of iron is [ar] 3d64s2. Iron is a transition metal with the atomic. Iron has 26 electrons in total. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Writing the electron configuration, you really only need the. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. In order to write the electron configuration for iron (fe) we first need to know the. Its valence orbitals are the 4s and 3d 's. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals.
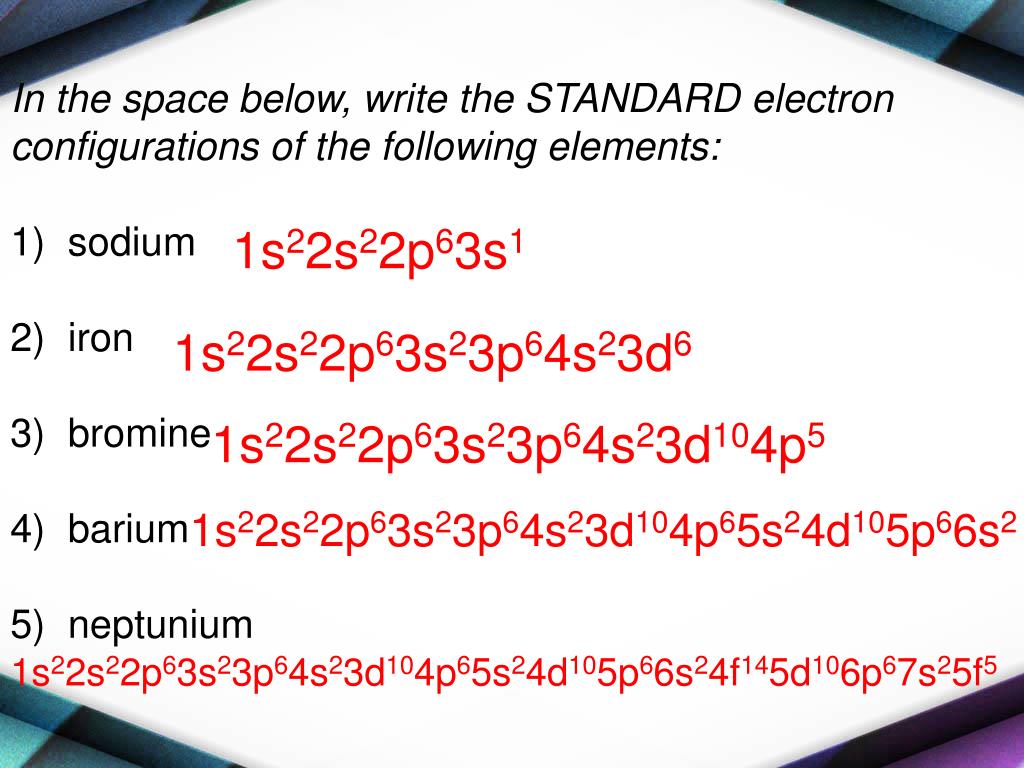
From ceguvkcr.blob.core.windows.net
What Kind Of Orbital Does Iron's Electron Configuration End With at What Is Electron Configuration For Iron In order to write the electron configuration for iron (fe) we first need to know the. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Iron is a transition metal with the atomic. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Its valence orbitals are the 4s. What Is Electron Configuration For Iron.
From electronschematic.blogspot.com
Electron Schematic What Is The Longhand Electron Configuration For Iron What Is Electron Configuration For Iron Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. In order to write the electron configuration for iron (fe) we first need to know the. It is termed as ferromagnetic materials. Iron has 26 electrons in total. The electron configuration of iron (fe) is [ar] 3d6 4s2. Iron is a chemical element of the periodic table. What Is Electron Configuration For Iron.
From ar.inspiredpencil.com
Electron Configuration Of Iron What Is Electron Configuration For Iron In order to write the electron configuration for iron (fe) we first need to know the. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Irons peculiar crystalline structure and. What Is Electron Configuration For Iron.
From www.toppr.com
Electronic Configuration of Iron Fe element Valency, Applications What Is Electron Configuration For Iron Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron has 26 electrons in total. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Iron is a transition metal with the atomic. Its valence orbitals. What Is Electron Configuration For Iron.
From www.toppr.com
The atomic number of iron is 26 . The electronic configuration is What Is Electron Configuration For Iron It is termed as ferromagnetic materials. Its valence orbitals are the 4s and 3d 's. Iron has 26 electrons in total. The electron configuration of iron (fe) is [ar] 3d6 4s2. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Its core orbitals are the 1s, 2s, 2p. What Is Electron Configuration For Iron.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image What Is Electron Configuration For Iron Its valence orbitals are the 4s and 3d 's. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. The electron configuration of iron (fe) is [ar] 3d6 4s2. In order to. What Is Electron Configuration For Iron.
From www.youtube.com
Electron Configuration of Transition Metals Iron Fe Cobalt Co and What Is Electron Configuration For Iron It is termed as ferromagnetic materials. In order to write the electron configuration for iron (fe) we first need to know the. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. The electron configuration of iron (fe) is [ar] 3d6 4s2. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Iron has. What Is Electron Configuration For Iron.
From www.youtube.com
A stepbystep description of how to write the electron configuration What Is Electron Configuration For Iron It is termed as ferromagnetic materials. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron has 26 electrons in total. The electron configuration of iron (fe) is [ar] 3d6 4s2.. What Is Electron Configuration For Iron.
From www.alamy.com
Iron (Fe). Diagram of the nuclear composition and electron What Is Electron Configuration For Iron Iron is a transition metal with the atomic. Its valence orbitals are the 4s and 3d 's. Iron has 26 electrons in total. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Writing the electron configuration, you really only need the. Iron is a chemical element of the periodic table with chemical symbol fe and atomic. What Is Electron Configuration For Iron.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration What Is Electron Configuration For Iron Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Iron has 26 electrons in total. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron is a. What Is Electron Configuration For Iron.
From new--electronic.blogspot.com
Electron Configuration Of Ferrous Ion What Is Electron Configuration For Iron Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. The electron configuration of iron (fe) is [ar] 3d6 4s2. Its valence orbitals are the 4s and 3d 's. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron is a transition metal with the. What Is Electron Configuration For Iron.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms What Is Electron Configuration For Iron Iron is a transition metal with the atomic. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Its valence orbitals are the 4s and 3d 's. It is termed as ferromagnetic materials. Irons peculiar crystalline. What Is Electron Configuration For Iron.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and What Is Electron Configuration For Iron The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Iron is a transition metal with the atomic. In order to write the. What Is Electron Configuration For Iron.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic What Is Electron Configuration For Iron It is termed as ferromagnetic materials. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron has 26 electrons in total. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Iron is a transition metal. What Is Electron Configuration For Iron.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is Electron Configuration For Iron Electronic configuration of iron is [ar] 3d64s2. Writing the electron configuration, you really only need the. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Iron has 26 electrons in total. In order to write the electron configuration for iron (fe) we first need to know the. It is termed as ferromagnetic materials. The electron configuration. What Is Electron Configuration For Iron.
From loehtqxsp.blob.core.windows.net
Iron Electron Configuration Class 10 at Jill Jackson blog What Is Electron Configuration For Iron Iron is a transition metal with the atomic. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Electronic configuration of iron is. What Is Electron Configuration For Iron.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet What Is Electron Configuration For Iron Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Electronic configuration of iron is [ar] 3d64s2. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. The electronic configuration of iron is a symbolic depiction of. What Is Electron Configuration For Iron.
From electronschematic.blogspot.com
Electron Schematic What Is The Longhand Electron Configuration For Iron What Is Electron Configuration For Iron The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron has 26 electrons in total. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. The electron configuration of iron (fe) is [ar] 3d6 4s2. Its core orbitals are the 1s,. What Is Electron Configuration For Iron.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) What Is Electron Configuration For Iron Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Writing the electron configuration, you really only need the. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Iron has 26 electrons in total. It is termed as ferromagnetic materials.. What Is Electron Configuration For Iron.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron What Is Electron Configuration For Iron Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Electronic configuration of iron is [ar] 3d64s2. In order to write the electron configuration for iron (fe) we first need to know the. Iron is a transition metal with the atomic. Iron has 26 electrons in total. The electron configuration of iron (fe) is [ar] 3d6 4s2.. What Is Electron Configuration For Iron.
From www.chegg.com
Solved What is the electron configuration of the Iron(III) What Is Electron Configuration For Iron Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. In order to write the electron configuration for iron (fe) we first need. What Is Electron Configuration For Iron.
From sciencenotes.org
List of Electron Configurations of Elements What Is Electron Configuration For Iron The electron configuration of iron (fe) is [ar] 3d6 4s2. It is termed as ferromagnetic materials. Its valence orbitals are the 4s and 3d 's. Iron is a transition metal with the atomic. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. In order to write the electron configuration for iron (fe) we first need to. What Is Electron Configuration For Iron.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration What Is Electron Configuration For Iron It is termed as ferromagnetic materials. The electron configuration of iron (fe) is [ar] 3d6 4s2. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Writing the electron configuration, you really only need the. Its valence orbitals are the 4s and 3d 's.. What Is Electron Configuration For Iron.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science What Is Electron Configuration For Iron Electronic configuration of iron is [ar] 3d64s2. Iron is a transition metal with the atomic. The electron configuration of iron (fe) is [ar] 3d6 4s2. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. It is termed as ferromagnetic materials. Iron is a chemical element of the periodic table with chemical symbol. What Is Electron Configuration For Iron.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) What Is Electron Configuration For Iron Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron is a transition metal with the atomic. Iron exhibits different types of. What Is Electron Configuration For Iron.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements What Is Electron Configuration For Iron Writing the electron configuration, you really only need the. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. It is termed as ferromagnetic materials. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron is. What Is Electron Configuration For Iron.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library What Is Electron Configuration For Iron Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Writing the electron configuration, you really only need the. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron exhibits different types. What Is Electron Configuration For Iron.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) What Is Electron Configuration For Iron Electronic configuration of iron is [ar] 3d64s2. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. The electron configuration of iron (fe) is [ar] 3d6 4s2. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron is a transition metal with the atomic. It. What Is Electron Configuration For Iron.
From ceguvkcr.blob.core.windows.net
What Kind Of Orbital Does Iron's Electron Configuration End With at What Is Electron Configuration For Iron In order to write the electron configuration for iron (fe) we first need to know the. It is termed as ferromagnetic materials. The electron configuration of iron (fe) is [ar] 3d6 4s2. Iron is a transition metal with the atomic. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Iron exhibits different types of allotropic forms. What Is Electron Configuration For Iron.
From www.haikudeck.com
Iron by Claudia Stevens What Is Electron Configuration For Iron In order to write the electron configuration for iron (fe) we first need to know the. Its valence orbitals are the 4s and 3d 's. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Iron has 26 electrons in total. Electronic configuration of iron is [ar] 3d64s2. Writing the electron configuration, you really only need the.. What Is Electron Configuration For Iron.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) What Is Electron Configuration For Iron Writing the electron configuration, you really only need the. The electron configuration of iron (fe) is [ar] 3d6 4s2. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Its valence orbitals are the 4s and 3d 's. It is termed as ferromagnetic materials. Iron has 26 electrons in total. Iron is a. What Is Electron Configuration For Iron.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions What Is Electron Configuration For Iron Its valence orbitals are the 4s and 3d 's. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. Iron is a transition metal with the atomic. Electronic configuration of iron is [ar] 3d64s2. It is termed as ferromagnetic materials. Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's.. What Is Electron Configuration For Iron.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron What Is Electron Configuration For Iron Its valence orbitals are the 4s and 3d 's. Iron is a transition metal with the atomic. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. The electronic configuration of iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Iron has 26 electrons in total. Writing the electron. What Is Electron Configuration For Iron.
From sciencenotes.org
Iron Atom Science Notes and Projects What Is Electron Configuration For Iron Its valence orbitals are the 4s and 3d 's. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. The electron configuration of iron (fe) is [ar] 3d6 4s2. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. In order to write the electron configuration for iron (fe) we. What Is Electron Configuration For Iron.
From ar.inspiredpencil.com
Electron Configuration Of Iron What Is Electron Configuration For Iron Its valence orbitals are the 4s and 3d 's. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Iron is a transition metal with the atomic. Writing the electron configuration,. What Is Electron Configuration For Iron.