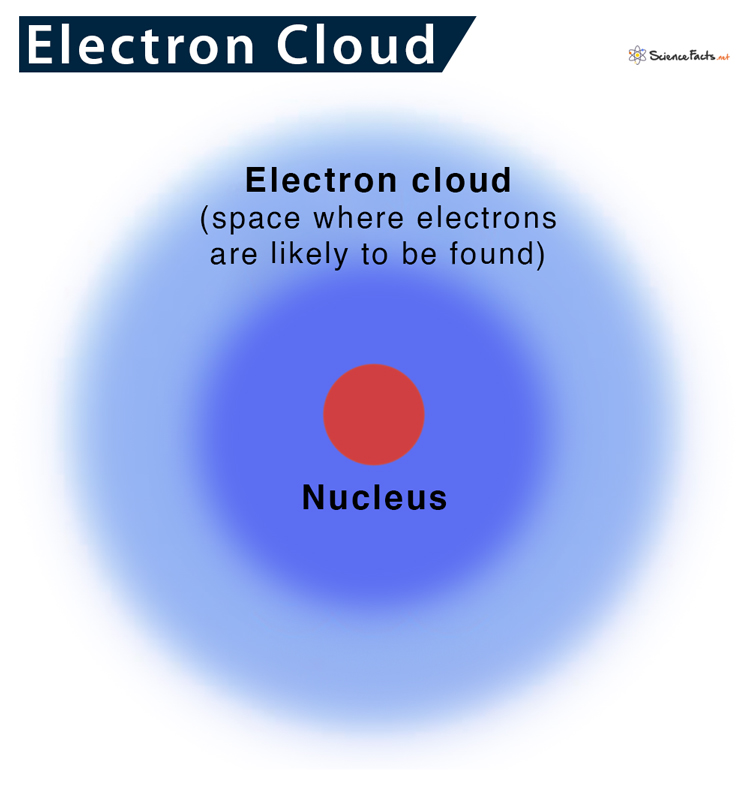
What Is Electron Cloud Shielding . The amount of charge felt by an. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. Because electrons in a particle have different extents. Electrons further from the nucleus (red) do not affect the z eff. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus.
from www.sciencefacts.net
Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The amount of charge felt by an. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. Electrons further from the nucleus (red) do not affect the z eff. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Because electrons in a particle have different extents.
Electron Cloud Definition and Diagram
What Is Electron Cloud Shielding The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons further from the nucleus (red) do not affect the z eff. Because electrons in a particle have different extents. The amount of charge felt by an. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of.
From www.universetoday.com
electron cloud model Archives Universe Today What Is Electron Cloud Shielding Because electrons in a particle have different extents. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. The amount of charge felt by an. Electrons further from the nucleus (red) do not affect the z eff. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full. What Is Electron Cloud Shielding.
From www.researchgate.net
(a) Simplified schematic of SERS process, (b) Polarized electrons cloud... Download Scientific What Is Electron Cloud Shielding According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of. What Is Electron Cloud Shielding.
From www.slideserve.com
PPT Electron Cloud Model PowerPoint Presentation, free download ID6119664 What Is Electron Cloud Shielding The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Electrons further from the nucleus (red) do not affect the z eff. Because electrons in a particle have different extents. The amount of charge felt by an. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full. What Is Electron Cloud Shielding.
From wisc.pb.unizin.org
Core and Valence Electrons, Shielding, Zeff (M7Q8) UWMadison Chemistry 103/104 Resource Book What Is Electron Cloud Shielding (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. Electrons further from the nucleus (red) do not. What Is Electron Cloud Shielding.
From www.zmescience.com
What is the Electron Cloud Model this is how electrons inside an atom really behave What Is Electron Cloud Shielding The amount of charge felt by an. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. The concept called electron shielding involves the outer electrons are partially shielded from the attractive. What Is Electron Cloud Shielding.
From www.researchgate.net
The electron cloud surrounding metals prevents incident light from... Download Scientific Diagram What Is Electron Cloud Shielding The amount of charge felt by an. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of. What Is Electron Cloud Shielding.
From ar.inspiredpencil.com
Electron Shielding What Is Electron Cloud Shielding The amount of charge felt by an. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons further from the nucleus (red) do not affect the z eff. Shielding effect can. What Is Electron Cloud Shielding.
From www.pinterest.com
Electron Cloud Theory Explained Science Struck Electrons, Clouds, Atom What Is Electron Cloud Shielding The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in. What Is Electron Cloud Shielding.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Engineering Projects What Is Electron Cloud Shielding (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The amount of charge felt by an. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. Because electrons in a particle have different extents. The concept called electron shielding involves the. What Is Electron Cloud Shielding.
From www.youtube.com
What is an electron cloud simple definition? YouTube What Is Electron Cloud Shielding Because electrons in a particle have different extents. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. The amount of charge. What Is Electron Cloud Shielding.
From www.slideserve.com
PPT Chapter 29 PowerPoint Presentation, free download ID6546371 What Is Electron Cloud Shielding The amount of charge felt by an. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. Electrons further from the nucleus (red) do not affect the z eff. Because electrons in a particle have different extents. (a) the. What Is Electron Cloud Shielding.
From www.researchgate.net
Atom and electron cloud (source Images) with nucleus in the... Download Scientific What Is Electron Cloud Shielding Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. Because electrons in a particle have different extents. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus.. What Is Electron Cloud Shielding.
From www.scienceabc.com
Electron Cloud Definition, Model, Explanation And Examples What Is Electron Cloud Shielding Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. Because electrons in a particle have different extents. Electrons further from the. What Is Electron Cloud Shielding.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is Electron Cloud Shielding Because electrons in a particle have different extents. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. The concept called electron. What Is Electron Cloud Shielding.
From www.youtube.com
electron cloud model YouTube What Is Electron Cloud Shielding Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Electrons further from the nucleus (red) do not affect the z eff.. What Is Electron Cloud Shielding.
From www.sliderbase.com
What is the Electron Cloud Model? Diagram What Is Electron Cloud Shielding The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. The amount of charge felt by an. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to. What Is Electron Cloud Shielding.
From www.slideserve.com
PPT Electron Arrangement PowerPoint Presentation, free download ID6502162 What Is Electron Cloud Shielding According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. The amount of charge felt by an. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The concept called electron shielding involves the outer electrons are partially shielded from the attractive. What Is Electron Cloud Shielding.
From www.researchgate.net
Quantum atomic model with electron cloud 6 Download Scientific Diagram What Is Electron Cloud Shielding Electrons further from the nucleus (red) do not affect the z eff. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. The amount of charge felt by an. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. What Is Electron Cloud Shielding.
From www.toppr.com
The ionization energies from Ga to Tl do not decrease due to What Is Electron Cloud Shielding (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons further from the nucleus (red) do not affect the z eff. The amount of charge felt by an. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Shielding effect can. What Is Electron Cloud Shielding.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting, and 5 Reliable Applications What Is Electron Cloud Shielding Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. Because electrons in a particle have different extents. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus.. What Is Electron Cloud Shielding.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Is Electron Cloud Shielding The amount of charge felt by an. Because electrons in a particle have different extents. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. (a) the interior electron cloud (light blue) shields the outer electron of interest from. What Is Electron Cloud Shielding.
From www.thoughtco.com
What Is an Electron Cloud? What Is Electron Cloud Shielding According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. Electrons further from the nucleus (red) do not affect the z eff. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Because electrons in a particle have different extents. Shielding effect can be defined as. What Is Electron Cloud Shielding.
From www.youtube.com
The Electron Cloud Model explained YouTube What Is Electron Cloud Shielding According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Electrons further from the nucleus (red) do not affect the z eff. (a) the interior electron cloud (light blue) shields the outer electron of interest from. What Is Electron Cloud Shielding.
From www.sciencefacts.net
Electron Cloud Definition and Diagram What Is Electron Cloud Shielding (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. The amount of charge felt by an. The. What Is Electron Cloud Shielding.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2617702 What Is Electron Cloud Shielding (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons further from the nucleus (red) do not affect the z eff. Because electrons in a particle have different extents. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to. What Is Electron Cloud Shielding.
From www.youtube.com
Electron shielding YouTube What Is Electron Cloud Shielding Because electrons in a particle have different extents. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. The amount of charge felt by an. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. Shielding effect can be defined as a reduction in the effective. What Is Electron Cloud Shielding.
From stock.adobe.com
Electron Cloud and important facts of the Electron Cloud Model Stock Vector Adobe Stock What Is Electron Cloud Shielding Because electrons in a particle have different extents. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. (a) the interior electron. What Is Electron Cloud Shielding.
From www.researchgate.net
An electron cloud model for contact electrification between two... Download Scientific Diagram What Is Electron Cloud Shielding Electrons further from the nucleus (red) do not affect the z eff. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. The amount of charge felt by an. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. According to the. What Is Electron Cloud Shielding.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding ShowMe What Is Electron Cloud Shielding Electrons further from the nucleus (red) do not affect the z eff. Because electrons in a particle have different extents. The amount of charge felt by an. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force. What Is Electron Cloud Shielding.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Electron Cloud Shielding Electrons further from the nucleus (red) do not affect the z eff. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The amount of charge felt by an. Because electrons in a particle have different extents. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther. What Is Electron Cloud Shielding.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity, Ionization Energy What Is Electron Cloud Shielding Shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces of the electrons on the nucleus. Because electrons in a particle have different extents. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Electrons further from the. What Is Electron Cloud Shielding.
From ar.inspiredpencil.com
Electron Cloud Definition What Is Electron Cloud Shielding Because electrons in a particle have different extents. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Electrons further from the nucleus (red) do not affect the z eff. The amount. What Is Electron Cloud Shielding.
From www.slideserve.com
PPT Understanding Flame Tests and Emission Spectra PowerPoint Presentation ID1562308 What Is Electron Cloud Shielding (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. Because electrons in a particle have different extents. The amount of charge felt by an. Electrons further from the nucleus (red) do not affect the z eff. Shielding effect can be defined as a reduction in the effective nuclear. What Is Electron Cloud Shielding.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is Electron Cloud Shielding The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of. Electrons further from the nucleus (red) do not affect the z eff. The amount of charge felt by an. Because electrons in a particle have different extents. Shielding effect can be defined as a reduction in the effective nuclear charge on the electron. What Is Electron Cloud Shielding.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download ID3646738 What Is Electron Cloud Shielding Electrons further from the nucleus (red) do not affect the z eff. According to the shielding effect, electrons nearer the nucleus “shield” electrons farther from the nucleus’ positive charge. (a) the interior electron cloud (light blue) shields the outer electron of interest from the full attractive force of the nucleus. The concept called electron shielding involves the outer electrons are. What Is Electron Cloud Shielding.