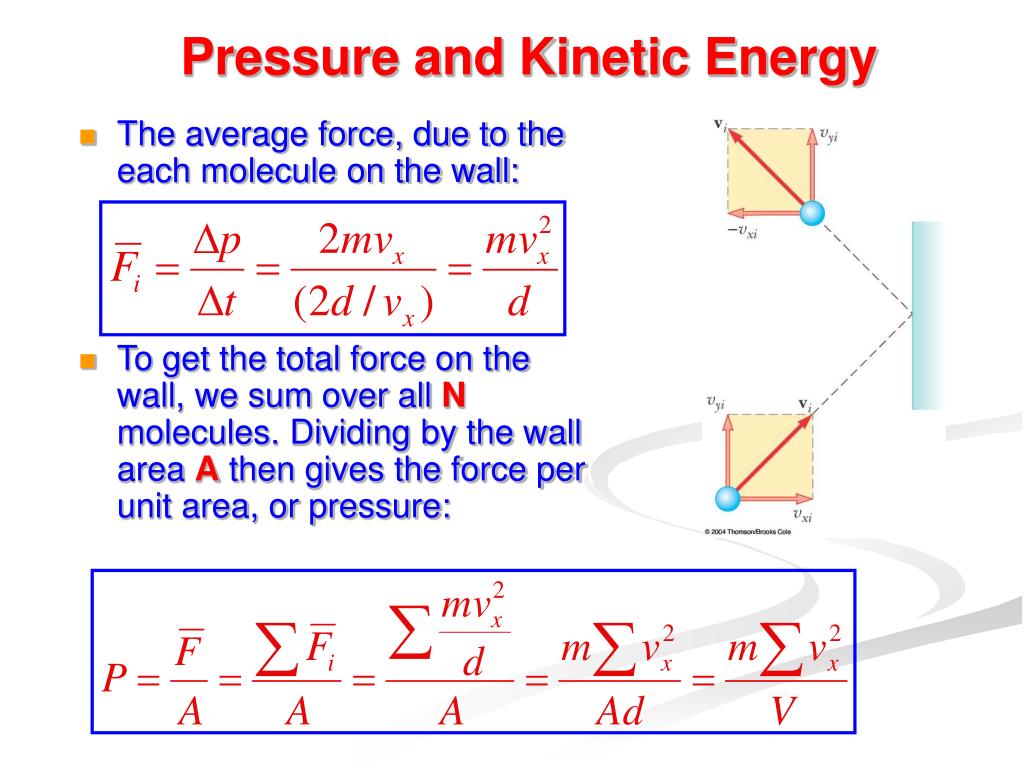
What Is Kinetic Pressure . The gas law links pressure,. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within.
from www.slideserve.com
pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. The gas law links pressure,.
PPT Chapter 21 PowerPoint Presentation, free download ID4316913
What Is Kinetic Pressure the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. The gas law links pressure,. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,.
From www.studypool.com
SOLUTION What is pressure and its derivation Studypool What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is the force divided by the area on which the force. What Is Kinetic Pressure.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint What Is Kinetic Pressure The gas law links pressure,. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. pressure is the force. What Is Kinetic Pressure.
From www.tec-science.com
Pressure and temperature theory of gases) tecscience What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. the behaviour of gases is described in terms of the kinetic theory, which. What Is Kinetic Pressure.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download ID1441785 What Is Kinetic Pressure learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. The gas law links pressure,. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. the behaviour of gases is described in terms of the kinetic theory, which considers the motion. What Is Kinetic Pressure.
From www.youtube.com
theory of Gases Assumption Introduction Derivation of What Is Kinetic Pressure the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. The gas law links pressure,. pressure is explained by kinetic theory as. What Is Kinetic Pressure.
From www.slideshare.net
Theory of Matter 2 Internal Energy & Pressure What Is Kinetic Pressure the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,.. What Is Kinetic Pressure.
From www.toppr.com
The relation between the gas pressure P and average energy per What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. learn about kinetic theory, which includes using the celsius and kelvin scales, the. What Is Kinetic Pressure.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID4316913 What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion. What Is Kinetic Pressure.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID4316913 What Is Kinetic Pressure learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting. What Is Kinetic Pressure.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID4316913 What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. The gas law links pressure,. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. pressure is the force divided by the. What Is Kinetic Pressure.
From socratic.org
What causes gas pressure (in terms of theory)? Socratic What Is Kinetic Pressure learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. The gas law links pressure,. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is explained by kinetic theory as arising from the force exerted by molecules or. What Is Kinetic Pressure.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,.. What Is Kinetic Pressure.
From www.slideserve.com
PPT Pressure and Energy PowerPoint Presentation, free What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. The gas law links pressure,. the behaviour of gases is described in. What Is Kinetic Pressure.
From www.slideserve.com
PPT Pressure and Energy PowerPoint Presentation, free What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. The gas law links pressure,. pressure is the force divided by the. What Is Kinetic Pressure.
From www.researchgate.net
EnergyPressure curve for blunt, conical and hemispherical What Is Kinetic Pressure use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. The gas law links pressure,. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is the force divided by the. What Is Kinetic Pressure.
From www.slideserve.com
PPT The Theory, Pressure & Gas Laws PowerPoint Presentation What Is Kinetic Pressure the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls. What Is Kinetic Pressure.
From www.youtube.com
Theory of Gases Pressure Derivation A Level Physics YouTube What Is Kinetic Pressure The gas law links pressure,. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. learn about kinetic theory, which includes using. What Is Kinetic Pressure.
From www.venkatsacademy.com
theory of gases and Expression for Pressure IIT JEE and NEET What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. The gas law links pressure,. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is the force divided by the. What Is Kinetic Pressure.
From www.pinterest.com.mx
A graphic showing the derivation of the dynamic pressure from the What Is Kinetic Pressure The gas law links pressure,. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. the behaviour of gases. What Is Kinetic Pressure.
From www.slideserve.com
PPT The Theory, Pressure & Gas Laws PowerPoint Presentation What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. use the gas laws and kinetic theory to relate the pressure, volume. What Is Kinetic Pressure.
From www.youtube.com
Using theory to explain gas pressure YouTube What Is Kinetic Pressure the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. What Is Kinetic Pressure.
From www.slideserve.com
PPT Theory and Gases PowerPoint Presentation, free download What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. The gas law links pressure,. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. pressure is explained by kinetic theory as arising from the force exerted by molecules or. What Is Kinetic Pressure.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID256534 What Is Kinetic Pressure the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. learn about kinetic theory, which includes using the celsius and kelvin scales,. What Is Kinetic Pressure.
From www.youtube.com
Interpretation of Pressure on Theory of Gases, Physics Lecture What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. pressure is the force divided by the area on which the force. What Is Kinetic Pressure.
From www.slideserve.com
PPT Pressure and Energy PowerPoint Presentation, free What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. the behaviour of gases is described in terms of the kinetic theory,. What Is Kinetic Pressure.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID256534 What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms. What Is Kinetic Pressure.
From studylib.net
Pressure and Energy What Is Kinetic Pressure The gas law links pressure,. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. pressure is the force divided by the area on which the force. What Is Kinetic Pressure.
From www.studypool.com
SOLUTION What is pressure and its derivation Studypool What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. pressure is the force divided by the area on which the force is. What Is Kinetic Pressure.
From www.studypool.com
SOLUTION What is pressure and its derivation Studypool What Is Kinetic Pressure The gas law links pressure,. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion of the molecules within. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. pressure is the force divided by the. What Is Kinetic Pressure.
From www.youtube.com
Derivation of relationship between energy per unit volume and What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. learn about kinetic theory, which includes using the celsius and kelvin scales, the. What Is Kinetic Pressure.
From www.tec-science.com
Pressure and temperature theory of gases) tecscience What Is Kinetic Pressure the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. What Is Kinetic Pressure.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID4316913 What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a gas to the motion. What Is Kinetic Pressure.
From www.youtube.com
Relationship between pressure and temperature (derivation, What Is Kinetic Pressure pressure is explained by kinetic theory as arising from the force exerted by molecules or atoms impacting on the walls of a container, as. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is the force divided by the area on which the force. What Is Kinetic Pressure.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID6041035 What Is Kinetic Pressure pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. use the gas laws and kinetic theory to relate the pressure, volume and temperature of a. What Is Kinetic Pressure.
From www.hotelsrate.org
Bernoulli Energy Equation With Pump Diy Projects What Is Kinetic Pressure learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure,. the behaviour of gases is described in terms of the kinetic theory, which considers the motion of molecules in the gas. pressure is the force divided by the area on which the force is exerted, and temperature is measured with a. What Is Kinetic Pressure.