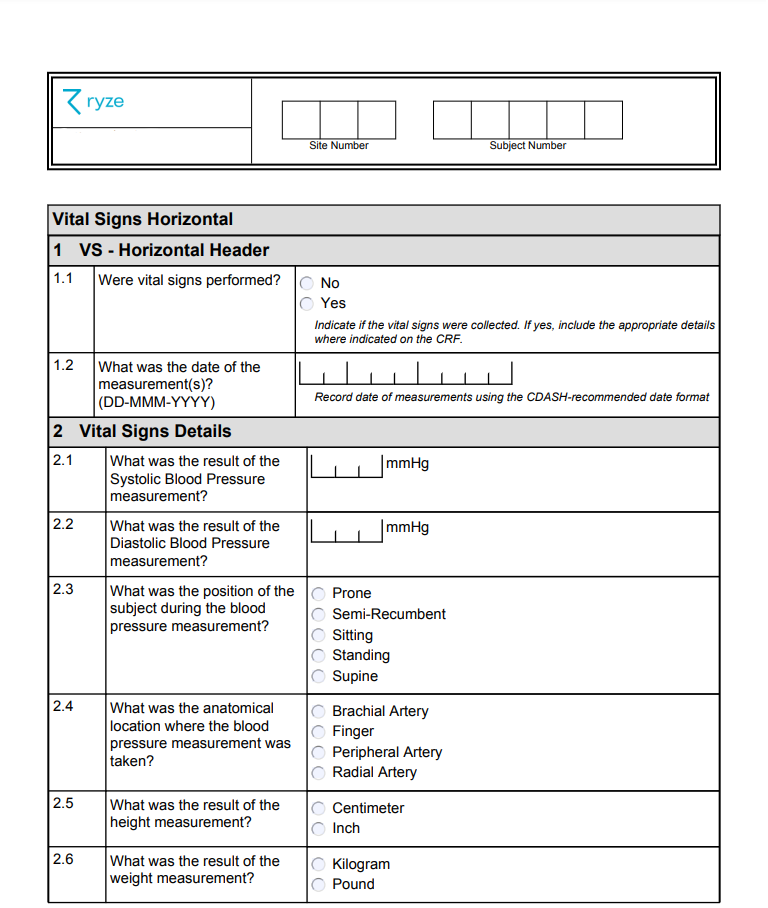
Case Report Form Clinical Trial Example . Its development represents a significant part of the clinical trial. Designing a case report form (crf) is vital for accurate clinical trial data collection. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It should align with the study protocol, regulatory. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. It defines crfs and explains that they contain all protocol. A case report form (crf) is designed to collect the patient data in a clinical trial;
from pharmaphorum.com
Designing a case report form (crf) is vital for accurate clinical trial data collection. Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It defines crfs and explains that they contain all protocol. A case report form (crf) is designed to collect the patient data in a clinical trial;
An introduction to case report forms pharmaphorum
Case Report Form Clinical Trial Example It defines crfs and explains that they contain all protocol. Its development represents a significant part of the clinical trial. A case report form (crf) is designed to collect the patient data in a clinical trial; It should align with the study protocol, regulatory. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. Designing a case report form (crf) is vital for accurate clinical trial data collection. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It defines crfs and explains that they contain all protocol.
From cloudflare.itsnudimension.com
Free Clinical Trial Templates Smartsheet Within Case Report Form Case Report Form Clinical Trial Example 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Its development represents a significant part of the clinical trial. It defines crfs and explains that they contain all protocol. It should align with the study protocol, regulatory. A case report form (crf) is. Case Report Form Clinical Trial Example.
From www.pinterest.com
Clinical Trial Report Template (3) TEMPLATES EXAMPLE TEMPLATES Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. It defines crfs and explains that they contain all protocol. Designing a case report form (crf) is vital for accurate clinical trial data collection. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined. Case Report Form Clinical Trial Example.
From pray.gelorailmu.com
Free 15+ Case Report Forms In Pdf Ms Word inside Case Report Form Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. A case report form (crf) is designed to collect the patient data in a clinical trial; It defines crfs and explains that they contain all protocol. It should align with the study protocol, regulatory. Its development represents a significant part of the clinical. Case Report Form Clinical Trial Example.
From newcreativetemplateideas.blogspot.com
Case Report Form Template Clinical Trials New Creative Template Ideas Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. Designing a case report form (crf) is vital for accurate clinical trial data collection. A case report form (crf) is designed to collect the patient data in a clinical trial; Its development represents a significant part of the clinical trial. The document discusses case report forms (crfs), which are used in clinical. Case Report Form Clinical Trial Example.
From businesstemplateinspiration.blogspot.com
Case Report Form Template Clinical Trials Case Report Form Clinical Trial Example It defines crfs and explains that they contain all protocol. Its development represents a significant part of the clinical trial. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. Designing a case report form (crf) is vital for accurate clinical trial data collection. A case report form (crf) is designed to collect. Case Report Form Clinical Trial Example.
From www.smartsheet.com
Free Clinical Trial Templates Smartsheet Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It defines crfs and explains that they contain all protocol. A case report form (crf) is designed to collect the patient data in a clinical trial;. Case Report Form Clinical Trial Example.
From documents.thegreenerleithsocial.org
Case Report Form Template Clinical Trials Documents Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. It should align with the study protocol, regulatory. Designing a case report form (crf) is vital for accurate clinical trial data collection. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the. Case Report Form Clinical Trial Example.
From bestprofessionaltemplate.blogspot.com
Clinical Trial Report Template Case Report Form Clinical Trial Example Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. It defines crfs and explains that they contain all protocol. A case report form (crf) is designed to collect the patient data in a clinical trial; The document discusses case report forms (crfs), which are used in clinical trials to record patient. Case Report Form Clinical Trial Example.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Its development represents a significant part of the clinical trial. The document discusses case report forms (crfs), which are used in clinical trials to record patient. Case Report Form Clinical Trial Example.
From www.altexsoft.com
Clinical Trial Software EDC, CTMS, ePRO, RTSM AltexSoft Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Designing a case report form (crf) is vital for accurate clinical trial data collection. A case report form (crf) is designed to collect the patient data. Case Report Form Clinical Trial Example.
From pharmaphorum.com
An introduction to case report forms pharmaphorum Case Report Form Clinical Trial Example A case report form (crf) is designed to collect the patient data in a clinical trial; The document discusses case report forms (crfs), which are used in clinical trials to record patient data. Its development represents a significant part of the clinical trial. It defines crfs and explains that they contain all protocol. It should align with the study protocol,. Case Report Form Clinical Trial Example.
From doctemplates.us
Case Report Form Template Clinical Trials DocTemplates Case Report Form Clinical Trial Example Its development represents a significant part of the clinical trial. A case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It defines crfs and explains that they contain. Case Report Form Clinical Trial Example.
From www.jotform.com
Medical Case Report PDF Templates Jotform Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. Its development represents a significant part of the clinical trial. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual. Case Report Form Clinical Trial Example.
From cloudflare.itsnudimension.com
Jmi A Good Clinical Trial Imaging throughout Case Case Report Form Clinical Trial Example 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It defines crfs and explains that they contain all protocol. Designing a case report form (crf) is vital for accurate clinical trial data collection. Its development represents a significant part of the clinical trial.. Case Report Form Clinical Trial Example.
From www.pinterest.com
The terrific Journalbasics Of Case Report Form Designing In Clinical Case Report Form Clinical Trial Example Designing a case report form (crf) is vital for accurate clinical trial data collection. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. The document. Case Report Form Clinical Trial Example.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial Example A case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. It. Case Report Form Clinical Trial Example.
From www.xfanzexpo.com
Case Eport Form Electronic Qolty Format Ppt In Clinical With Trial Case Report Form Clinical Trial Example Designing a case report form (crf) is vital for accurate clinical trial data collection. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It should align. Case Report Form Clinical Trial Example.
From www.smartsheet.com
Free Clinical Trial Templates Smartsheet Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Designing a case report form (crf) is vital for accurate clinical trial data collection. The document discusses case report forms (crfs), which are used in clinical. Case Report Form Clinical Trial Example.
From designtemplatework.blogspot.com
Case Report Form Template Clinical Trials Free Design Template for Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. A case report form (crf) is designed to collect the patient data in a clinical trial; Designing a case report form (crf) is vital for. Case Report Form Clinical Trial Example.
From designtemplatework.blogspot.com
Case Report Form Template Clinical Trials Free Design Template for Case Report Form Clinical Trial Example Designing a case report form (crf) is vital for accurate clinical trial data collection. Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. A case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used. Case Report Form Clinical Trial Example.
From support.theboogaloo.org
Pertussis Case Report Form Queensland Health In Case Report Form Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. A case report form (crf) is designed to collect the patient data in a clinical trial; The document discusses case report forms (crfs), which are used in clinical trials to record patient data. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined. Case Report Form Clinical Trial Example.
From pray.gelorailmu.com
Pro Forma Document (Case Report Form) Used To Record The With Case Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. It defines crfs and explains that they contain all protocol. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Designing a case report form (crf) is. Case Report Form Clinical Trial Example.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial Example Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. Designing a case report form (crf) is vital for accurate clinical trial data collection. A case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used. Case Report Form Clinical Trial Example.
From support.theboogaloo.org
Free 15+ Case Report Forms In Pdf Ms Word for Case Report Form Case Report Form Clinical Trial Example Designing a case report form (crf) is vital for accurate clinical trial data collection. It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Its development represents a significant part of the clinical trial. The document. Case Report Form Clinical Trial Example.
From www.sampletemplate.my.id
Monitoring Report Template Clinical Trials Sampletemplate.my.id Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. It should align with the study protocol, regulatory. A case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined. Case Report Form Clinical Trial Example.
From cloudflare.itsnudimension.com
Free Clinical Trial Templates Smartsheet Regarding Case Report Form Case Report Form Clinical Trial Example A case report form (crf) is designed to collect the patient data in a clinical trial; It defines crfs and explains that they contain all protocol. Designing a case report form (crf) is vital for accurate clinical trial data collection. It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool used. Case Report Form Clinical Trial Example.
From pray.gelorailmu.com
Free 15+ Case Report Forms In Pdf Ms Word pertaining to Case Report Case Report Form Clinical Trial Example A case report form (crf) is designed to collect the patient data in a clinical trial; Designing a case report form (crf) is vital for accurate clinical trial data collection. It defines crfs and explains that they contain all protocol. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. 1.2 the case. Case Report Form Clinical Trial Example.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial Example Designing a case report form (crf) is vital for accurate clinical trial data collection. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It should align. Case Report Form Clinical Trial Example.
From www.scientific.net
Based Case Report Form Design for Clinical Trial Case Report Form Clinical Trial Example It should align with the study protocol, regulatory. A case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Designing a case report form (crf) is vital for accurate. Case Report Form Clinical Trial Example.
From pray.gelorailmu.com
Free 15+ Case Report Forms In Pdf Ms Word In Case Report Form Case Report Form Clinical Trial Example A case report form (crf) is designed to collect the patient data in a clinical trial; It should align with the study protocol, regulatory. Its development represents a significant part of the clinical trial. The document discusses case report forms (crfs), which are used in clinical trials to record patient data. It defines crfs and explains that they contain all. Case Report Form Clinical Trial Example.
From www.sampletemplates.com
FREE 11+ Clinical Audit Report Templates in PDF MS Word Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. A case report form (crf) is designed to collect the patient data in a clinical trial; Its development represents a significant part of the clinical trial. Designing a case report form (crf) is vital for accurate clinical trial data collection. 1.2 the case. Case Report Form Clinical Trial Example.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Clinical Trial Example Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. Designing a case report form (crf) is vital for accurate clinical trial data collection. A case report form (crf) is designed to collect the patient data in a clinical trial; 1.2 the case report form (crf) is a data collection tool used. Case Report Form Clinical Trial Example.
From templatedbc.web.app
Crf Case Report Form Templates PrintableDB.web.app Case Report Form Clinical Trial Example Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It defines crfs and explains that they contain all protocol. The document discusses case report forms. Case Report Form Clinical Trial Example.
From businesstemplateinspiration.blogspot.com
Case Report Form Template Clinical Trials Case Report Form Clinical Trial Example The document discusses case report forms (crfs), which are used in clinical trials to record patient data. Designing a case report form (crf) is vital for accurate clinical trial data collection. Its development represents a significant part of the clinical trial. It should align with the study protocol, regulatory. 1.2 the case report form (crf) is a data collection tool. Case Report Form Clinical Trial Example.
From www.semanticscholar.org
Basics of case report form designing in clinical research Semantic Case Report Form Clinical Trial Example 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. It should align with the study protocol, regulatory. Its development represents a significant part of the clinical trial. The document discusses case report forms (crfs), which are used in clinical trials to record patient. Case Report Form Clinical Trial Example.