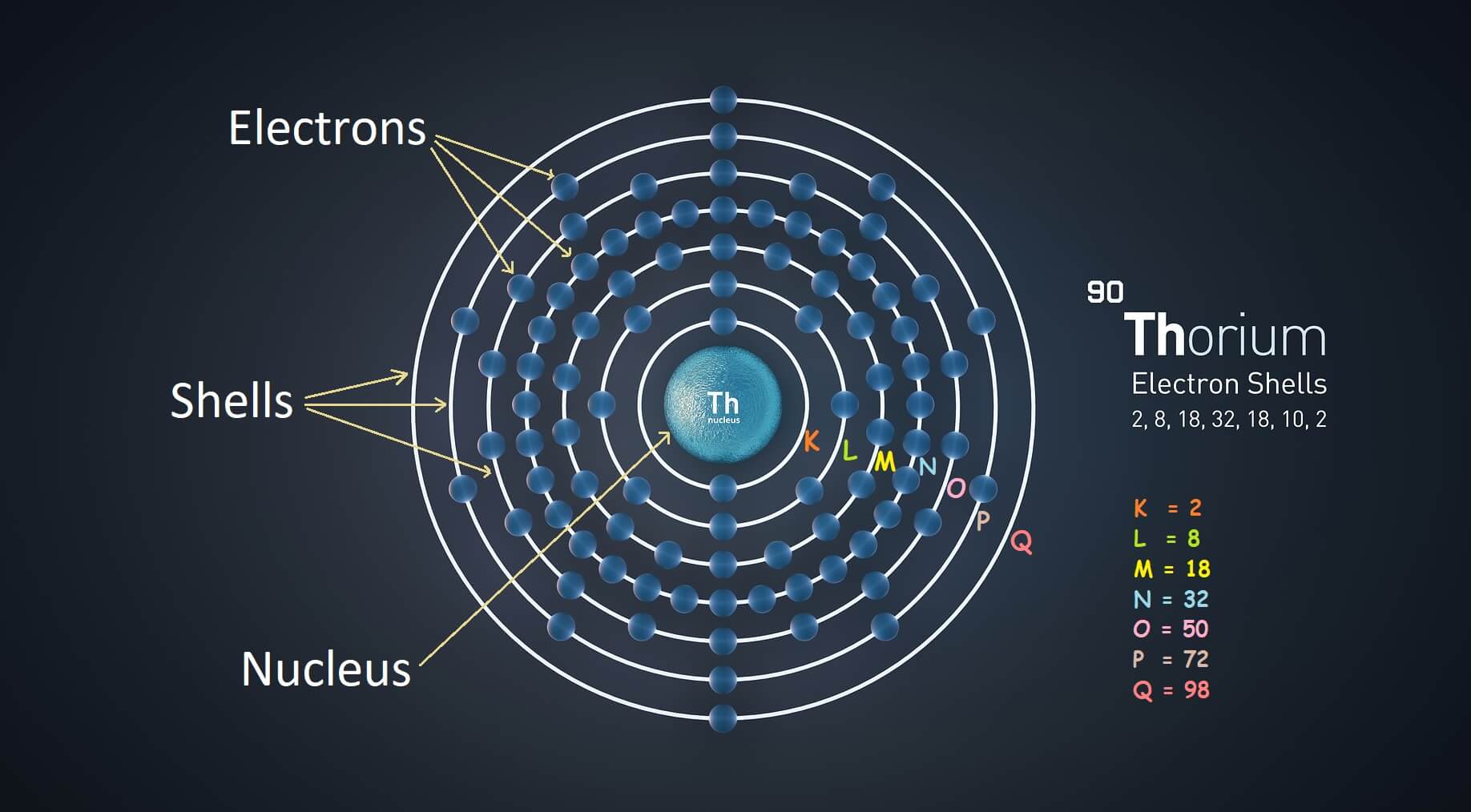
Iron Electrons Shell . The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. As a result, iron has eight valence electrons. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The closest shell to the nucleus is. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. 119 rows electron configuration chart of all elements is mentioned in the table below. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The shorthand electron configuration (or noble gas configuration) as well as.
from newtondesk.com
The closest shell to the nucleus is. The shorthand electron configuration (or noble gas configuration) as well as. As a result, iron has eight valence electrons. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. 119 rows electron configuration chart of all elements is mentioned in the table below. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively.
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry
Iron Electrons Shell As a result, iron has eight valence electrons. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. As a result, iron has eight valence electrons. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. 119 rows electron configuration chart of all elements is mentioned in the table below. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The closest shell to the nucleus is. The shorthand electron configuration (or noble gas configuration) as well as.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Electrons Shell This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The shorthand electron configuration (or noble gas configuration) as well as. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. The closest shell to the. Iron Electrons Shell.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Electrons Shell As a result, iron has eight valence electrons. The closest shell to the nucleus is. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. The shorthand electron configuration (or noble gas configuration) as well as.. Iron Electrons Shell.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo Bigstock Iron Electrons Shell In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. 119 rows electron configuration chart of all elements is mentioned. Iron Electrons Shell.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Iron Electrons Shell The shorthand electron configuration (or noble gas configuration) as well as. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. 119 rows electron configuration chart of all elements is mentioned in the table below. The closest shell to the nucleus is. The reason being that there are three different subshells in the third. Iron Electrons Shell.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free Iron Electrons Shell Iron atoms have 26 electrons and the shell structure is 2.8.14.2. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. 119 rows electron configuration chart of all elements is mentioned in the table below. The. Iron Electrons Shell.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Electrons Shell Iron atoms have 26 electrons and the shell structure is 2.8.14.2. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. As a result, iron has eight valence electrons. The shorthand electron configuration (or noble gas configuration) as well as. The ground state electron configuration of ground. Iron Electrons Shell.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Electrons Shell The shorthand electron configuration (or noble gas configuration) as well as. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. 119 rows electron configuration chart of all elements is mentioned in the table below. As a result, iron has eight valence electrons. In chemistry and atomic physics, an electron shell may be thought. Iron Electrons Shell.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Iron Electrons Shell The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. This configuration shows that iron has two electrons in its outermost shell, making it. Iron Electrons Shell.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron Electrons Shell 119 rows electron configuration chart of all elements is mentioned in the table below. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. The closest shell to the nucleus is. The ground state electron configuration. Iron Electrons Shell.
From byjus.com
Electronic Configuration How To Write Electron ConfigurationChemistry Iron Electrons Shell 119 rows electron configuration chart of all elements is mentioned in the table below. The closest shell to the nucleus is. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus.. Iron Electrons Shell.
From chemistrytalk.org
Electron Shells ChemTalk Iron Electrons Shell In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. This configuration shows that iron has two electrons in. Iron Electrons Shell.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Electrons Shell The closest shell to the nucleus is. 119 rows electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. In chemistry and atomic physics, an electron shell may be. Iron Electrons Shell.
From ceaulups.blob.core.windows.net
Iron Has How Many Electrons In The Shells at Nicole Paige blog Iron Electrons Shell The shorthand electron configuration (or noble gas configuration) as well as. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. 119 rows electron. Iron Electrons Shell.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image Iron Electrons Shell In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has eight valence electrons. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The reason. Iron Electrons Shell.
From justanothershop-a-holic.blogspot.com
Electron Configuration Of Iron Full worksheet today Iron Electrons Shell 119 rows electron configuration chart of all elements is mentioned in the table below. The closest shell to the nucleus is. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively.. Iron Electrons Shell.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Electrons Shell As a result, iron has eight valence electrons. The closest shell to the nucleus is. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. 119 rows electron configuration chart of all elements is mentioned in the table below. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which. Iron Electrons Shell.
From awesomehome.co
Iron Periodic Table Electrons Awesome Home Iron Electrons Shell The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. As a result, iron has eight valence electrons. The reason being that there are three different subshells in the third shell (called s, p and d. Iron Electrons Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Iron Electrons Shell As a result, iron has eight valence electrons. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The shorthand electron configuration (or noble gas configuration) as well as. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. 119 rows electron configuration chart of all. Iron Electrons Shell.
From slcc.pressbooks.pub
4.2 The Structure of Atoms College Biology I Iron Electrons Shell The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. 119 rows electron configuration chart of all elements is mentioned in the table below. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The reason being that there are three different subshells in the third. Iron Electrons Shell.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Iron Electrons Shell The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The closest shell to the nucleus is. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has eight valence electrons.. Iron Electrons Shell.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Iron Electrons Shell The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. The shorthand electron configuration (or noble gas configuration) as well as. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. 119 rows electron configuration chart of all elements. Iron Electrons Shell.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Electrons Shell The shorthand electron configuration (or noble gas configuration) as well as. As a result, iron has eight valence electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The reason being that there are three different subshells in the third shell (called s, p. Iron Electrons Shell.
From rapidelectron.blogspot.com
Electron Configuration For An Atom Of Iron Rapid Electron Iron Electrons Shell The closest shell to the nucleus is. 119 rows electron configuration chart of all elements is mentioned in the table below. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. As a result, iron has eight valence electrons. Iron atoms have 26 electrons and the shell. Iron Electrons Shell.
From www.dreamstime.com
Iron Atom Stock Illustrations 1,568 Iron Atom Stock Illustrations Iron Electrons Shell As a result, iron has eight valence electrons. The closest shell to the nucleus is. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. The shorthand electron configuration (or noble gas configuration) as well as. Iron atoms have 26 electrons and the shell structure is 2.8.14.2.. Iron Electrons Shell.
From ceaulups.blob.core.windows.net
Iron Has How Many Electrons In The Shells at Nicole Paige blog Iron Electrons Shell The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. The reason being. Iron Electrons Shell.
From autoctrls.com
The Fascinating Structure of Electron Shells A Diagrammatic Exploration Iron Electrons Shell Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The ground state electron configuration. Iron Electrons Shell.
From www.alamy.com
Iron atom hires stock photography and images Alamy Iron Electrons Shell The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. The shorthand electron configuration (or noble gas configuration) as well as. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom. Iron Electrons Shell.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Electrons Shell In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. 119 rows electron configuration chart of all elements is mentioned in the table below. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2,. Iron Electrons Shell.
From www.alamy.com
Iron (Fe). Diagram of the nuclear composition and electron Iron Electrons Shell The closest shell to the nucleus is. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. As a result, iron has eight valence electrons. The shorthand electron configuration (or noble gas configuration) as well as. 119 rows electron configuration chart of all elements is mentioned in. Iron Electrons Shell.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Electrons Shell The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. This configuration shows. Iron Electrons Shell.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Electrons Shell The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. This configuration shows that iron has two electrons in its outermost shell, making it. Iron Electrons Shell.
From www.scienceabc.com
Noble Metal Definition, List, Properties, And Examples Iron Electrons Shell This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has. Iron Electrons Shell.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electrons Shell The shorthand electron configuration (or noble gas configuration) as well as. The reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons, respectively. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The closest shell to the nucleus is. In chemistry and. Iron Electrons Shell.
From www.alamy.com
Fe Iron, Periodic Table of the Elements, Shell Structure of Iron Iron Electrons Shell The closest shell to the nucleus is. 119 rows electron configuration chart of all elements is mentioned in the table below. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. This configuration shows that iron has two electrons in its outermost shell, making it highly reactive. As a result, iron has eight valence electrons. The shorthand electron configuration. Iron Electrons Shell.
From www.worldanvil.com
Iron Material in The Spaceport World Anvil Iron Electrons Shell The ground state electron configuration of ground state gaseous neutral iron is [ar].3d 6.4s 2 and the term. The shorthand electron configuration (or noble gas configuration) as well as. 119 rows electron configuration chart of all elements is mentioned in the table below. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The reason being that there are. Iron Electrons Shell.