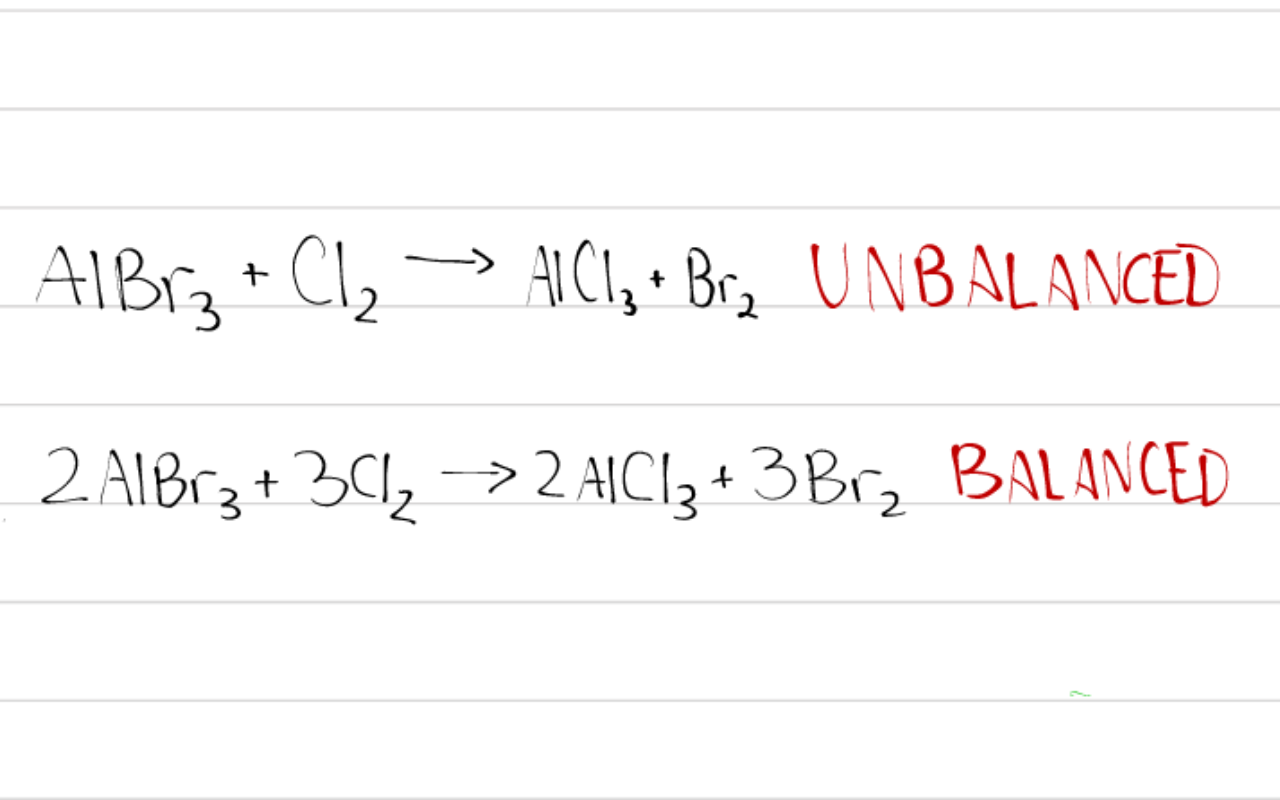What Is The Formula Of Bromine . The chemical element bromine is classed as a halogen and a nonmetal. Bromine forms compounds in many oxidation states: A single covalent bond connecting the atoms. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Seven valence electrons per atom, fourteen in total for br₂. It has an atomic weight of 79.904 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It was discovered in 1825 by carl lðwig (loewig). Widely used in flame retardants and certain types of medication. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5.
from ar.inspiredpencil.com
Bromine forms compounds in many oxidation states: Seven valence electrons per atom, fourteen in total for br₂. A single covalent bond connecting the atoms. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It has an atomic weight of 79.904 and a mass. Widely used in flame retardants and certain types of medication. The chemical element bromine is classed as a halogen and a nonmetal. The most stable oxidation state of the element is −1, in which bromine occurs naturally.
Bromine Liquid Formula
What Is The Formula Of Bromine Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It was discovered in 1825 by carl lðwig (loewig). Widely used in flame retardants and certain types of medication. Bromine forms compounds in many oxidation states: It has an atomic weight of 79.904 and a mass. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. A single covalent bond connecting the atoms. Seven valence electrons per atom, fourteen in total for br₂. The chemical element bromine is classed as a halogen and a nonmetal. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5.
From www.pw.live
Bromine Formula, Valency, Mass And Properties What Is The Formula Of Bromine It has an atomic weight of 79.904 and a mass. Bromine forms compounds in many oxidation states: The most stable oxidation state of the element is −1, in which bromine occurs naturally. A single covalent bond connecting the atoms. Seven valence electrons per atom, fourteen in total for br₂. It was discovered in 1825 by carl lðwig (loewig). Sources, facts,. What Is The Formula Of Bromine.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector What Is The Formula Of Bromine The chemical element bromine is classed as a halogen and a nonmetal. Seven valence electrons per atom, fourteen in total for br₂. Bromine forms compounds in many oxidation states: Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It has an atomic weight of 79.904 and a mass. It. What Is The Formula Of Bromine.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector What Is The Formula Of Bromine Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Seven valence electrons per atom, fourteen in total for br₂. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. A single covalent bond. What Is The Formula Of Bromine.
From www.alamy.com
Bromine element hires stock photography and images Alamy What Is The Formula Of Bromine A single covalent bond connecting the atoms. Seven valence electrons per atom, fourteen in total for br₂. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Widely used in flame retardants and certain types of medication. The chemical element bromine is classed as a halogen and a nonmetal. Bromine forms compounds in many oxidation states: Bromine is the 35th element in. What Is The Formula Of Bromine.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine What Is The Formula Of Bromine But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It was discovered in 1825 by carl lðwig (loewig). Seven valence electrons per atom, fourteen in total for br₂. It has an atomic weight of 79.904 and a mass. A single covalent bond connecting. What Is The Formula Of Bromine.
From stock.adobe.com
Vector ballandstick model of chemical substance. Dark red icon of What Is The Formula Of Bromine Bromine forms compounds in many oxidation states: The chemical element bromine is classed as a halogen and a nonmetal. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Widely used in flame retardants and certain types of medication. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite,. What Is The Formula Of Bromine.
From www.alamy.com
Bromine molecule, illustration Stock Photo Alamy What Is The Formula Of Bromine The most stable oxidation state of the element is −1, in which bromine occurs naturally. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. A single covalent bond connecting the atoms. Bromine forms compounds in many oxidation states: It was discovered in 1825. What Is The Formula Of Bromine.
From www.youtube.com
How to Write the Formula for Bromine Liquid YouTube What Is The Formula Of Bromine Bromine forms compounds in many oxidation states: Widely used in flame retardants and certain types of medication. A single covalent bond connecting the atoms. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It has an atomic weight of 79.904 and a mass. The chemical element. What Is The Formula Of Bromine.
From molekula.com
Purchase Bromine [7726956] online • Catalog • Molekula Group What Is The Formula Of Bromine Widely used in flame retardants and certain types of medication. It has an atomic weight of 79.904 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It was discovered in 1825 by carl lðwig (loewig). But oxidation states. What Is The Formula Of Bromine.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds What Is The Formula Of Bromine The most stable oxidation state of the element is −1, in which bromine occurs naturally. It has an atomic weight of 79.904 and a mass. Widely used in flame retardants and certain types of medication. Seven valence electrons per atom, fourteen in total for br₂. It was discovered in 1825 by carl lðwig (loewig). Bromine is the 35th element in. What Is The Formula Of Bromine.
From ar.inspiredpencil.com
Bromine Liquid Formula What Is The Formula Of Bromine Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The chemical element bromine is classed as a halogen and a nonmetal. The most stable oxidation state of the element is −1, in which bromine occurs naturally. It was discovered in 1825 by carl lðwig (loewig). It has an atomic. What Is The Formula Of Bromine.
From www.shutterstock.com
Reaction Bromine Propene Chemical Structure Vector Vector có sẵn (miễn What Is The Formula Of Bromine Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Seven valence electrons per atom, fourteen in total for br₂. It has an atomic weight of 79.904 and a mass. Sources, facts,. What Is The Formula Of Bromine.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock What Is The Formula Of Bromine Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. A single covalent bond connecting the atoms. The chemical element bromine is classed as a halogen and a nonmetal.. What Is The Formula Of Bromine.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy What Is The Formula Of Bromine Widely used in flame retardants and certain types of medication. Seven valence electrons per atom, fourteen in total for br₂. The most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine is the 35th element in the. What Is The Formula Of Bromine.
From www.alamy.com
Bromine atom, with mass and energy levels. Vector illustration Stock What Is The Formula Of Bromine Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Widely used in flame retardants and certain types of medication. A single covalent bond connecting the atoms. Bromine forms compounds in many oxidation states: It has an atomic weight of 79.904 and a mass. It was discovered in 1825 by carl lðwig (loewig). Seven valence electrons per atom, fourteen in total for. What Is The Formula Of Bromine.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds What Is The Formula Of Bromine Widely used in flame retardants and certain types of medication. The chemical element bromine is classed as a halogen and a nonmetal. Seven valence electrons per atom, fourteen in total for br₂. It has an atomic weight of 79.904 and a mass. It was discovered in 1825 by carl lðwig (loewig). A single covalent bond connecting the atoms. The most. What Is The Formula Of Bromine.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock What Is The Formula Of Bromine It was discovered in 1825 by carl lðwig (loewig). Bromine forms compounds in many oxidation states: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The chemical element bromine is classed as a halogen and a nonmetal. It has an atomic. What Is The Formula Of Bromine.
From www.alamy.com
3d render of atom structure of bromine isolated over white background What Is The Formula Of Bromine Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It was discovered in 1825 by carl lðwig (loewig). A single covalent bond connecting the atoms. The chemical element bromine is classed as a halogen and a nonmetal. The most stable oxidation state of the element is −1, in which bromine occurs naturally. It has an atomic weight of 79.904 and a. What Is The Formula Of Bromine.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine What Is The Formula Of Bromine Widely used in flame retardants and certain types of medication. It was discovered in 1825 by carl lðwig (loewig). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine forms compounds in many oxidation states: Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The chemical element bromine is classed. What Is The Formula Of Bromine.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image What Is The Formula Of Bromine Bromine forms compounds in many oxidation states: A single covalent bond connecting the atoms. Seven valence electrons per atom, fourteen in total for br₂. It has an atomic weight of 79.904 and a mass. The most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite,. What Is The Formula Of Bromine.
From www.alamy.com
3D image of Bromine azide skeletal formula molecular chemical What Is The Formula Of Bromine Bromine forms compounds in many oxidation states: The most stable oxidation state of the element is −1, in which bromine occurs naturally. It was discovered in 1825 by carl lðwig (loewig). Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It has an atomic weight of 79.904 and a. What Is The Formula Of Bromine.
From www.alamy.com
Elemental bromine (Br2) molecule. Skeletal formula Stock Vector Image What Is The Formula Of Bromine Bromine forms compounds in many oxidation states: A single covalent bond connecting the atoms. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The chemical element bromine is classed as a halogen and a nonmetal. Widely used in flame retardants and certain types of medication. But oxidation states of. What Is The Formula Of Bromine.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector What Is The Formula Of Bromine It has an atomic weight of 79.904 and a mass. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Seven valence electrons per atom, fourteen in total for br₂. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Sources, facts, uses, scarcity (sri), podcasts, alchemical. What Is The Formula Of Bromine.
From testbook.com
Bromine Formula Learn Definition, Formula, Structure and Uses What Is The Formula Of Bromine Seven valence electrons per atom, fourteen in total for br₂. The most stable oxidation state of the element is −1, in which bromine occurs naturally. The chemical element bromine is classed as a halogen and a nonmetal. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Sources, facts, uses, scarcity (sri),. What Is The Formula Of Bromine.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica What Is The Formula Of Bromine Seven valence electrons per atom, fourteen in total for br₂. The chemical element bromine is classed as a halogen and a nonmetal. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. A single covalent bond connecting the atoms. It was discovered in 1825 by carl lðwig (loewig). Bromine is the 35th. What Is The Formula Of Bromine.
From www.alamy.com
Bromine molecule Br2 Stock Photo Alamy What Is The Formula Of Bromine It was discovered in 1825 by carl lðwig (loewig). A single covalent bond connecting the atoms. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. It has an atomic weight of 79.904 and a mass. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Sources,. What Is The Formula Of Bromine.
From www.vecteezy.com
Bromine symbol. Chemical element of the periodic table. Vector What Is The Formula Of Bromine Widely used in flame retardants and certain types of medication. The chemical element bromine is classed as a halogen and a nonmetal. Bromine forms compounds in many oxidation states: It has an atomic weight of 79.904 and a mass. A single covalent bond connecting the atoms. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It was discovered in 1825 by. What Is The Formula Of Bromine.
From gradegorilla.com
GCSE Chemistry Organic Grade Gorilla What Is The Formula Of Bromine But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine forms compounds in many oxidation states: Widely used in flame retardants and certain types of medication. It has an atomic weight of 79.904 and a mass. A single covalent bond connecting the atoms. Bromine is the 35th element in the periodic. What Is The Formula Of Bromine.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses What Is The Formula Of Bromine Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Seven valence electrons per atom, fourteen in total for br₂. The chemical element bromine is classed as a halogen and a nonmetal. It has an atomic weight of 79.904 and a mass. Widely used in flame retardants. What Is The Formula Of Bromine.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses What Is The Formula Of Bromine Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Seven valence electrons per atom, fourteen in total for br₂. It has an atomic weight of 79.904 and a mass. Bromine forms compounds in many oxidation states: It was discovered in 1825. What Is The Formula Of Bromine.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID2813065 What Is The Formula Of Bromine The chemical element bromine is classed as a halogen and a nonmetal. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Seven valence electrons per atom, fourteen in total for br₂. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is the 35th element in the periodic table and has a symbol of br. What Is The Formula Of Bromine.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image What Is The Formula Of Bromine Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine forms compounds in many oxidation states: A single covalent bond connecting the atoms. It was discovered in 1825 by carl lðwig (loewig). The chemical element bromine is classed as a halogen and a nonmetal. It has an atomic weight of 79.904 and a mass. The most stable oxidation state of the. What Is The Formula Of Bromine.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) What Is The Formula Of Bromine Bromine forms compounds in many oxidation states: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Widely used in flame retardants and certain types of medication. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Seven valence electrons per atom, fourteen in total for br₂. A single covalent bond connecting. What Is The Formula Of Bromine.
From material-properties.org
Bromine Periodic Table and Atomic Properties What Is The Formula Of Bromine A single covalent bond connecting the atoms. Seven valence electrons per atom, fourteen in total for br₂. Widely used in flame retardants and certain types of medication. It has an atomic weight of 79.904 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The chemical element bromine is classed as a halogen and a nonmetal. Bromine forms compounds. What Is The Formula Of Bromine.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration What Is The Formula Of Bromine It has an atomic weight of 79.904 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The most stable oxidation state of the element is −1, in which bromine occurs naturally. It was discovered in 1825 by carl lðwig (loewig). Bromine forms compounds in many oxidation states: A single covalent bond connecting the atoms. The chemical element bromine. What Is The Formula Of Bromine.