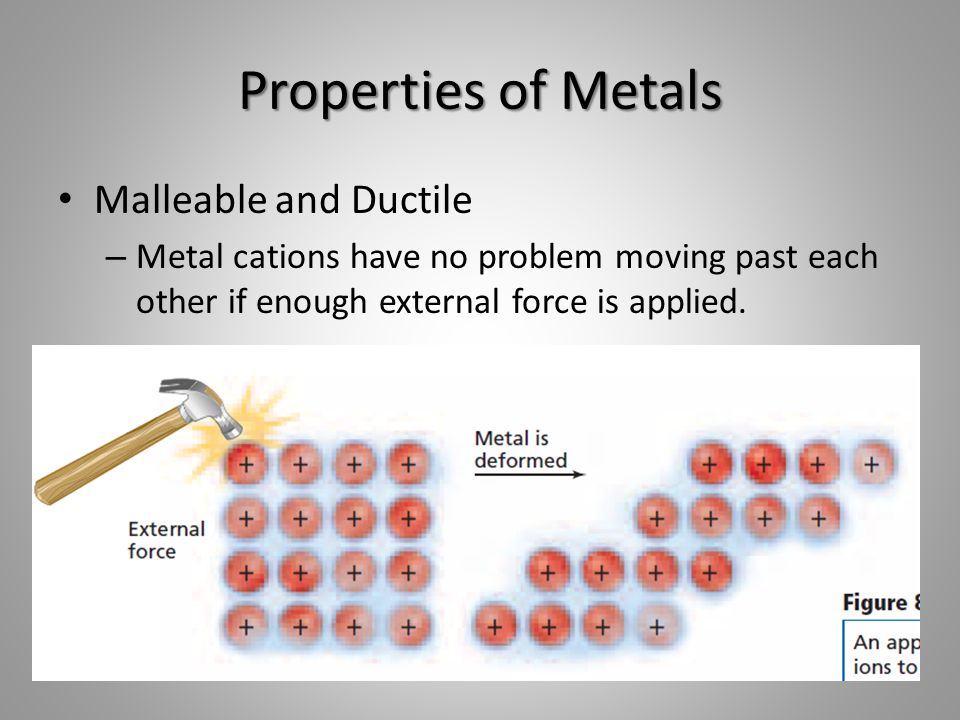
Are Malleable Metals Brittle . Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. They are poor conductors of heat and electricity. Gases (oxygen) and solids (carbon). First, note that crystals (and metals and ceramics are. It is quite wrong to suggest that all metals are malleable. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. Are in general more malleable. It is usually easy to tell metals and non. They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied. Metallic bonding allows the metal to change.
from slideplayer.com
They are poor conductors of heat and electricity. It is quite wrong to suggest that all metals are malleable. It is usually easy to tell metals and non. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. First, note that crystals (and metals and ceramics are. They are poor conductors of heat and electricity. Are in general more malleable. Gases (oxygen) and solids (carbon). Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c.
Metallic Bonds. ppt download
Are Malleable Metals Brittle They are poor conductors of heat and electricity. Are in general more malleable. They are poor conductors of heat and electricity. It is quite wrong to suggest that all metals are malleable. They are poor conductors of heat and electricity. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. First, note that crystals (and metals and ceramics are. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Metallic bonding allows the metal to change. Gases (oxygen) and solids (carbon). Metals are malleable because layers of ions can slide over each other when a force is applied. It is usually easy to tell metals and non.
From www.slideshare.net
Unit 3 Physical Properties of Matter Are Malleable Metals Brittle They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied. It is usually easy to tell metals and non. Are in general more malleable. Gases (oxygen) and solids (carbon). First, note that crystals (and metals and ceramics are. Let's draw a comparison with ceramics, which—just. Are Malleable Metals Brittle.
From studylib.net
Metals Are Malleable Metals Brittle Metallic bonding allows the metal to change. It is quite wrong to suggest that all metals are malleable. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Metals are malleable because layers of ions can slide over each other when a force is applied. They are poor conductors of heat and electricity. Iridium is an. Are Malleable Metals Brittle.
From byjus.com
Why are metals malleable, ductile, sonorus, lustrous and why are group Are Malleable Metals Brittle It is usually easy to tell metals and non. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Gases (oxygen) and solids (carbon). They are poor conductors of heat and electricity. First, note that crystals (and metals and ceramics are. It is quite wrong to suggest that all. Are Malleable Metals Brittle.
From ar.inspiredpencil.com
Malleability Examples Are Malleable Metals Brittle Gases (oxygen) and solids (carbon). They are poor conductors of heat and electricity. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. They are poor conductors of heat and electricity. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. First, note that crystals. Are Malleable Metals Brittle.
From slideplayer.com
Chemistry Change & Matter ppt download Are Malleable Metals Brittle It is quite wrong to suggest that all metals are malleable. They are poor conductors of heat and electricity. First, note that crystals (and metals and ceramics are. Metallic bonding allows the metal to change. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. Iridium is an elemental metal with the. Are Malleable Metals Brittle.
From slideplayer.com
The Modern Periodic Table ppt download Are Malleable Metals Brittle Gases (oxygen) and solids (carbon). First, note that crystals (and metals and ceramics are. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. They are poor conductors of heat and. Are Malleable Metals Brittle.
From slideplayer.com
Chapter 2 Properties of Matter ppt download Are Malleable Metals Brittle Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. Metallic bonding allows the metal to change. Gases (oxygen) and solids (carbon). It is quite wrong to suggest that all metals are malleable. They are poor conductors of heat and electricity. It is usually easy to tell metals and non. First, note. Are Malleable Metals Brittle.
From ar.inspiredpencil.com
Malleability Examples Are Malleable Metals Brittle First, note that crystals (and metals and ceramics are. Metallic bonding allows the metal to change. Are in general more malleable. They are poor conductors of heat and electricity. It is quite wrong to suggest that all metals are malleable. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. Metals are. Are Malleable Metals Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Malleable Metals Brittle It is usually easy to tell metals and non. They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied. Metallic bonding allows the metal to change. First, note that crystals (and metals and ceramics are. Iridium is an elemental metal with the same crystal structure. Are Malleable Metals Brittle.
From paris-yersblogbrown.blogspot.com
What Is the Most Malleable Metal Are Malleable Metals Brittle It is quite wrong to suggest that all metals are malleable. Metallic bonding allows the metal to change. They are poor conductors of heat and electricity. Are in general more malleable. First, note that crystals (and metals and ceramics are. It is usually easy to tell metals and non. Some intermetallic compounds, such as al 3 ti, feal, tial and. Are Malleable Metals Brittle.
From slideplayer.com
Physical Science Chemical Reactions—Day 21 Materials Needed ppt download Are Malleable Metals Brittle Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. First, note that crystals (and metals and ceramics are. It is usually easy to tell metals and non. They are poor conductors of heat and electricity. Gases (oxygen) and solids (carbon). It is quite wrong to suggest that all metals are malleable. Some intermetallic compounds, such. Are Malleable Metals Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Malleable Metals Brittle Metals are malleable because layers of ions can slide over each other when a force is applied. Are in general more malleable. They are poor conductors of heat and electricity. Gases (oxygen) and solids (carbon). They are poor conductors of heat and electricity. First, note that crystals (and metals and ceramics are. It is usually easy to tell metals and. Are Malleable Metals Brittle.
From www.slideshare.net
Physical properties Are Malleable Metals Brittle Are in general more malleable. It is usually easy to tell metals and non. They are poor conductors of heat and electricity. Gases (oxygen) and solids (carbon). Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above. Are Malleable Metals Brittle.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download Are Malleable Metals Brittle Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Are in general more malleable. Gases (oxygen) and solids (carbon). First, note that crystals (and metals and ceramics are. They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied. It is. Are Malleable Metals Brittle.
From www.slideserve.com
PPT Chapter 6 / Unit 2 Section A Periodic Table of elements Are Malleable Metals Brittle It is quite wrong to suggest that all metals are malleable. They are poor conductors of heat and electricity. Metallic bonding allows the metal to change. Metals are malleable because layers of ions can slide over each other when a force is applied. First, note that crystals (and metals and ceramics are. Gases (oxygen) and solids (carbon). Iridium is an. Are Malleable Metals Brittle.
From slideplayer.com
Physical Properties (Section 2.2) ppt download Are Malleable Metals Brittle Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Metals are malleable because layers of ions can slide over each other when a force is applied. First, note that crystals (and metals and ceramics are. Gases (oxygen) and solids (carbon). They are poor conductors of heat and electricity.. Are Malleable Metals Brittle.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1462875 Are Malleable Metals Brittle Metallic bonding allows the metal to change. Are in general more malleable. Gases (oxygen) and solids (carbon). It is quite wrong to suggest that all metals are malleable. Metals are malleable because layers of ions can slide over each other when a force is applied. They are poor conductors of heat and electricity. Let's draw a comparison with ceramics, which—just. Are Malleable Metals Brittle.
From slideplayer.com
KS4 Chemistry Metallic Bonding. ppt download Are Malleable Metals Brittle They are poor conductors of heat and electricity. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. It is usually easy to tell metals and non. Are in general more malleable. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. It is quite wrong to suggest. Are Malleable Metals Brittle.
From www.exampleslab.com
15 Examples of Malleable Materials Examples Lab Are Malleable Metals Brittle First, note that crystals (and metals and ceramics are. Metallic bonding allows the metal to change. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Gases (oxygen) and solids (carbon). They are poor conductors. Are Malleable Metals Brittle.
From www.slideserve.com
PPT 4.4 Metallic bonding PowerPoint Presentation ID400526 Are Malleable Metals Brittle Are in general more malleable. It is quite wrong to suggest that all metals are malleable. Gases (oxygen) and solids (carbon). Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. First, note that crystals (and metals and ceramics are. They are poor conductors of heat and electricity. It is usually easy to tell metals and. Are Malleable Metals Brittle.
From www.kapilsteels.com
Malleability in Metals A Complete Overview Are Malleable Metals Brittle Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. First, note that crystals (and metals and ceramics are. It is quite wrong to suggest that all metals are malleable. Gases (oxygen) and solids (carbon). Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at. Are Malleable Metals Brittle.
From slideplayer.com
Chapter 4 Atoms and Elements ppt download Are Malleable Metals Brittle Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Metals are malleable because layers of ions can slide over each other when a force is applied. It is quite wrong to suggest that all metals are malleable.. Are Malleable Metals Brittle.
From slidetodoc.com
Metallic Bonds Metallic Bonds Properties Bond Formation e Are Malleable Metals Brittle Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Metallic bonding allows the metal to change. They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied. It is quite wrong to suggest that. Are Malleable Metals Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Are Malleable Metals Brittle First, note that crystals (and metals and ceramics are. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. Are in general more malleable. Gases (oxygen) and solids (carbon). It is usually easy to tell. Are Malleable Metals Brittle.
From mymetalsgame.com
Is gold malleable or brittle? My Metals Game Are Malleable Metals Brittle Are in general more malleable. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. It is quite wrong to suggest that all metals are malleable. They are poor conductors of heat and electricity. It is usually easy to tell metals and non. Some intermetallic compounds, such as al. Are Malleable Metals Brittle.
From slideplayer.com
Chapter 7 “Ionic and Metallic Bonding” ppt download Are Malleable Metals Brittle Metals are malleable because layers of ions can slide over each other when a force is applied. First, note that crystals (and metals and ceramics are. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Are in general more malleable. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle. Are Malleable Metals Brittle.
From slideplayer.com
Unit 1 Chemistry (P ) Patterns and Compounds ppt download Are Malleable Metals Brittle It is usually easy to tell metals and non. Are in general more malleable. Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. Metals are malleable because layers of ions can slide over each other when a force is applied. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile. Are Malleable Metals Brittle.
From slideplayer.com
Metallic Bonds. ppt download Are Malleable Metals Brittle They are poor conductors of heat and electricity. First, note that crystals (and metals and ceramics are. It is usually easy to tell metals and non. Metals are malleable because layers of ions can slide over each other when a force is applied. Gases (oxygen) and solids (carbon). Metallic bonding allows the metal to change. It is quite wrong to. Are Malleable Metals Brittle.
From slideplayer.com
Bellwork Chapter Pretest ppt download Are Malleable Metals Brittle Are in general more malleable. It is usually easy to tell metals and non. They are poor conductors of heat and electricity. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. It is quite wrong to suggest that all metals are malleable. They are poor conductors of heat and electricity. Let's. Are Malleable Metals Brittle.
From www.youtube.com
Metals are Malleable and Ductile NonMetals Brittle YouTube Are Malleable Metals Brittle Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. It is quite wrong to suggest that all metals are malleable. Gases (oxygen) and solids (carbon). Metals are malleable because layers of ions can slide over each other when a force is applied. Let's draw a comparison with ceramics, which—just as metals. Are Malleable Metals Brittle.
From techiescientist.com
Is Copper Malleable? Techiescientist Are Malleable Metals Brittle Gases (oxygen) and solids (carbon). Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. First, note that crystals (and metals and ceramics are. They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied.. Are Malleable Metals Brittle.
From slideplayer.com
Metallic Bonds. ppt download Are Malleable Metals Brittle First, note that crystals (and metals and ceramics are. Metallic bonding allows the metal to change. Gases (oxygen) and solids (carbon). They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are. Are Malleable Metals Brittle.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Malleable Metals Brittle Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. They are poor conductors of heat and electricity. First, note that crystals (and metals and ceramics are. Gases (oxygen) and solids (carbon). Are in general more malleable. Metallic bonding allows the metal to change. They are poor conductors of heat and electricity. It is quite wrong. Are Malleable Metals Brittle.
From www.slideserve.com
PPT Worksheet Chapter 12 Homework Key PowerPoint Presentation, free Are Malleable Metals Brittle They are poor conductors of heat and electricity. Metals are malleable because layers of ions can slide over each other when a force is applied. Iridium is an elemental metal with the same crystal structure as aluminum that becomes ductile only at temperatures above 700 °c. They are poor conductors of heat and electricity. Metallic bonding allows the metal to. Are Malleable Metals Brittle.
From slideplayer.com
The Periodic Table. ppt download Are Malleable Metals Brittle They are poor conductors of heat and electricity. Gases (oxygen) and solids (carbon). Let's draw a comparison with ceramics, which—just as metals are generally ductile—are generally brittle. It is quite wrong to suggest that all metals are malleable. Some intermetallic compounds, such as al 3 ti, feal, tial and mosi 2, are brittle at room temperature. First, note that crystals. Are Malleable Metals Brittle.