Calorimeter Constant Equation Units . One technique we can use to measure the amount of heat. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. Calorimetry is used to measure amounts of heat transferred to or from a substance. The calibration gives you a number called the calorimeter constant. To do so, the heat is exchanged with a calibrated object. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree.
from www.youtube.com
Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. The calibration gives you a number called the calorimeter constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat.
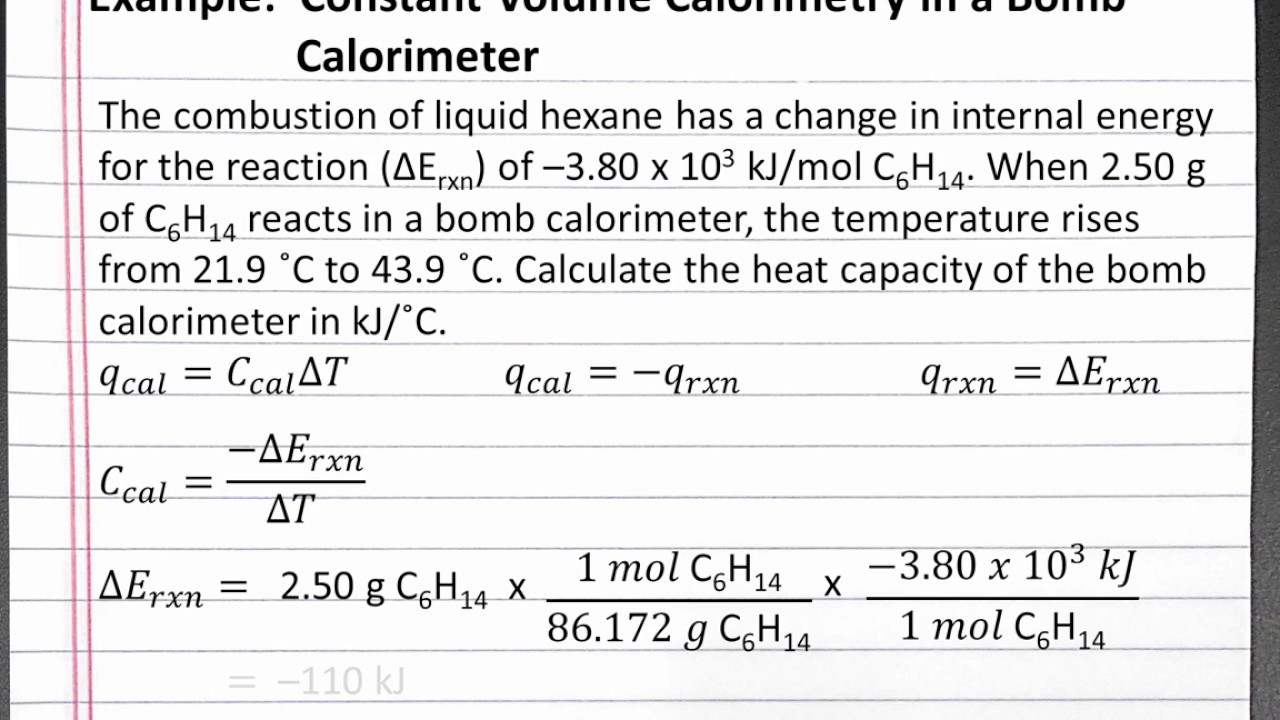
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube
Calorimeter Constant Equation Units Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Apply the first law of thermodynamics to calorimetry. The calibration gives you a number called the calorimeter constant. One technique we can use to measure the amount of heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimeter Constant Equation Units Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is used to measure amounts of heat transferred to or from a substance. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. To do so, the heat is exchanged with a calibrated object. Calculate and interpret heat and related. Calorimeter Constant Equation Units.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Calorimeter Constant Equation Units Apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. One technique we can use to measure the amount of heat. Compare heat flow from hot. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1875569 Calorimeter Constant Equation Units Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. To do so, the heat is exchanged with a calibrated object. Calculate and interpret heat and related properties using typical calorimetry data. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The calibration gives you a number called the calorimeter. Calorimeter Constant Equation Units.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Constant Equation Units One technique we can use to measure the amount of heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's. Calorimeter Constant Equation Units.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Calorimeter Constant Equation Units One technique we can use to measure the amount of heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. To do so, the heat is exchanged with a calibrated object. It's the amount of heat energy required to raise the. Calorimeter Constant Equation Units.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimeter Constant Equation Units Calculate and interpret heat and related properties using typical calorimetry data. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Apply the first law of thermodynamics to calorimetry. The calibration gives you a number called the calorimeter constant. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree.. Calorimeter Constant Equation Units.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Constant Equation Units Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. To do so, the heat is exchanged with a calibrated object. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. It's the amount of heat energy required to raise the temperature of. Calorimeter Constant Equation Units.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Constant Equation Units Calorimetry is used to measure amounts of heat transferred to or from a substance. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. To do so, the heat is exchanged with. Calorimeter Constant Equation Units.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimeter Constant Equation Units To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to. Calorimeter Constant Equation Units.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Constant Equation Units To do so, the heat is exchanged with a calibrated object. The calibration gives you a number called the calorimeter constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is used to measure amounts of heat transferred to or from a substance. Because \(δh\) is defined as the heat flow at constant. Calorimeter Constant Equation Units.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Constant Equation Units Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. The calibration gives you a number called the calorimeter constant. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to measure the amount of heat. Compare heat flow from hot to. Calorimeter Constant Equation Units.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Calorimeter Constant Equation Units It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. One technique we can use to measure. Calorimeter Constant Equation Units.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant Equation Units The calibration gives you a number called the calorimeter constant. Apply the first law of thermodynamics to calorimetry. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Calorimetry is used to measure amounts of heat transferred to or from a substance. It may be calculated by applying a known amount of heat. Calorimeter Constant Equation Units.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Calorimeter Constant Equation Units Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in.. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Equation Units It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. One technique we can use to measure the amount of heat. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Calorimeter Constant Equation Units It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. One technique we can use to measure the amount of heat. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Apply the first law of thermodynamics to calorimetry. The calibration gives you a number. Calorimeter Constant Equation Units.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Constant Equation Units Apply the first law of thermodynamics to calorimetry. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is used to measure amounts of heat transferred. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Equation Units Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. It may be calculated by applying a known amount of heat to the. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT UNIT 3 THERMOCHEMISTRY PowerPoint Presentation, free download Calorimeter Constant Equation Units One technique we can use to measure the amount of heat. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Calculate and interpret heat and related properties using typical calorimetry data. It may be calculated by applying a. Calorimeter Constant Equation Units.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimeter Constant Equation Units Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat. Calculate and interpret heat and related properties using typical calorimetry data. It may be calculated by applying a known amount. Calorimeter Constant Equation Units.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Calorimeter Constant Equation Units The calibration gives you a number called the calorimeter constant. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calculate and interpret heat and related properties using typical calorimetry data. One technique we. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Calorimeter Constant Equation Units It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. To do. Calorimeter Constant Equation Units.
From www.chegg.com
Calculate the calorimeter constant (From Lab) So for Calorimeter Constant Equation Units One technique we can use to measure the amount of heat. Calculate and interpret heat and related properties using typical calorimetry data. Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Because \(δh\) is defined as. Calorimeter Constant Equation Units.
From www.youtube.com
Calorimeter Constant YouTube Calorimeter Constant Equation Units Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in.. Calorimeter Constant Equation Units.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Calorimeter Constant Equation Units Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Calorimetry is used to measure amounts of heat transferred to or from a substance. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real.. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant Equation Units Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat. Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from. Calorimeter Constant Equation Units.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimeter Constant Equation Units Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. It's the amount of heat energy required to raise the temperature of the calorimeter by 1. Calorimeter Constant Equation Units.
From www.chegg.com
Solved The reaction below takes place in a constant pressure Calorimeter Constant Equation Units Calculate and interpret heat and related properties using typical calorimetry data. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It's. Calorimeter Constant Equation Units.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Constant Equation Units One technique we can use to measure the amount of heat. To do so, the heat is exchanged with a calibrated object. The calibration gives you a number called the calorimeter constant. Apply the first law of thermodynamics to calorimetry. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change. Calorimeter Constant Equation Units.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Constant Equation Units Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. The calibration gives you a number called the calorimeter constant. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. To do so, the heat is exchanged with a calibrated object. It may be calculated by applying a known. Calorimeter Constant Equation Units.
From www.youtube.com
09.05 Calorimetry YouTube Calorimeter Constant Equation Units Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat. It may be calculated by applying a known amount of heat to the. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Equation Units Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It's the amount of heat energy required to raise the temperature of the calorimeter by 1 degree. Apply the first law of thermodynamics to calorimetry. One technique we can use to. Calorimeter Constant Equation Units.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Constant Equation Units Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. The calibration gives you a number called the calorimeter constant. Because \(δh\) is defined as the. Calorimeter Constant Equation Units.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Constant Equation Units Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calculate and interpret heat and related properties using typical calorimetry data. It may be calculated by applying a known amount of heat to. Calorimeter Constant Equation Units.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure Calorimeter Constant Equation Units Because \(δh\) is defined as the heat flow at constant pressure, measurements made using. Calorimetry is used to measure amounts of heat transferred to or from a substance. It may be calculated by applying a known amount of heat to the calorimeter and measuring the calorimeter's corresponding change in. One technique we can use to measure the amount of heat.. Calorimeter Constant Equation Units.