Heating Curve Of Naphthalene . Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The particles are very closely. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. The experiment described above can be summarized in a graph called a heating curve (figure below). Naphthalene is in solid state at any temperature below its melting point. The sample is initially ice at 1 atm. The graph above shows the heating curve of naphthalene.
from www.numerade.com
The graph above shows the heating curve of naphthalene. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The sample is initially ice at 1 atm. Naphthalene is in solid state at any temperature below its melting point. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The experiment described above can be summarized in a graph called a heating curve (figure below). The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. The particles are very closely. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt.
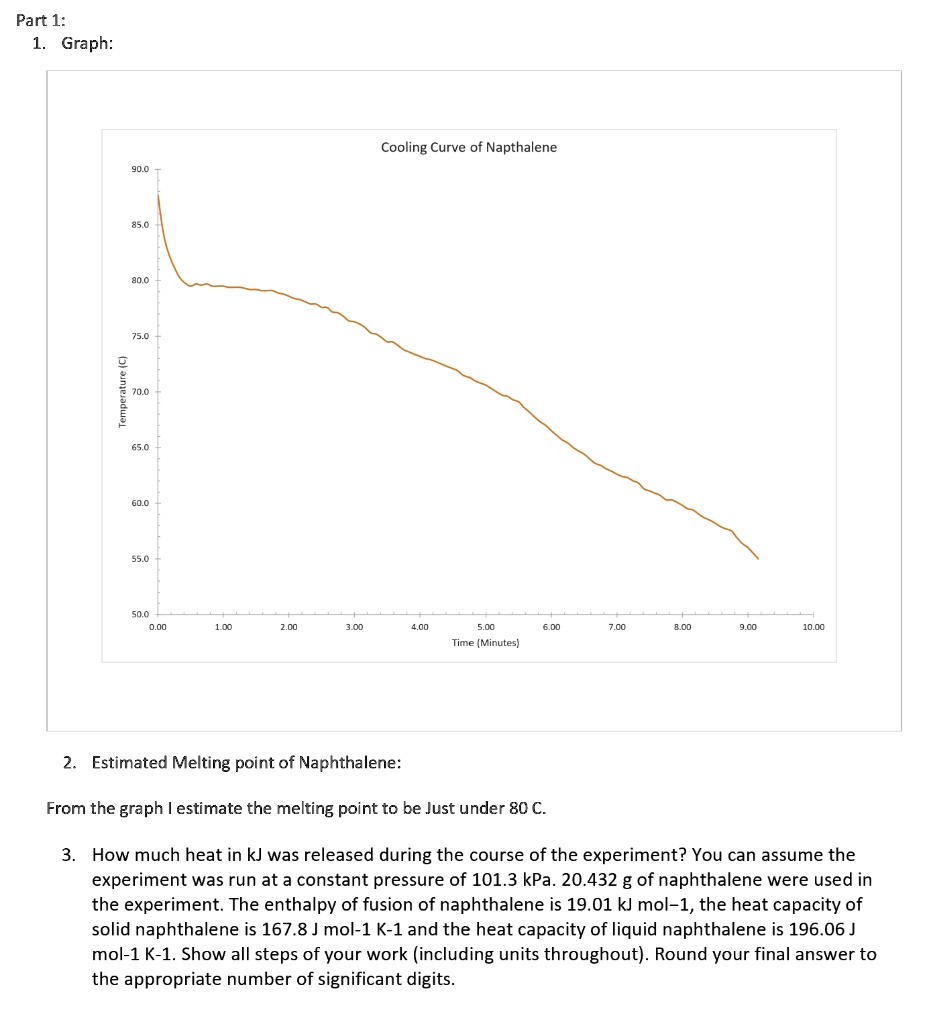
SOLVED Part 1 Graph Cooling Curve of Napthalene 1 1.00 10 Q6 Time
Heating Curve Of Naphthalene Naphthalene is in solid state at any temperature below its melting point. The experiment described above can be summarized in a graph called a heating curve (figure below). Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. Naphthalene is in solid state at any temperature below its melting point. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. The particles are very closely. The graph above shows the heating curve of naphthalene. The sample is initially ice at 1 atm. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water.
From www.researchgate.net
Standard curve of Naphthalene solution. Download Scientific Diagram Heating Curve Of Naphthalene The sample is initially ice at 1 atm. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The graph above. Heating Curve Of Naphthalene.
From www.researchgate.net
Computed and experimental mole fraction solubility ln x of naphthalene Heating Curve Of Naphthalene Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Naphthalene is in solid state at any temperature below its melting point. The particles are very closely. The sample is initially ice at 1 atm. The experiment described above can be summarized in a graph. Heating Curve Of Naphthalene.
From www.youtube.com
SPM Chemistry Form 4 Heating and cooling of Naphthalene YouTube Heating Curve Of Naphthalene Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. The sample is. Heating Curve Of Naphthalene.
From www.thinkswap.com
SPM Physics Experiment Heating and cooling of naphthalene Physics Heating Curve Of Naphthalene The graph above shows the heating curve of naphthalene. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2. Heating Curve Of Naphthalene.
From www.showme.com
Cooling curve of naphthalene lab Science, Chemistry ShowMe Heating Curve Of Naphthalene The particles are very closely. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Heating curve of naphthalene when naphthalene is heated, the temperature. Heating Curve Of Naphthalene.
From www.chegg.com
Solved Cooling curve of pure naphthalene Cooling graph of Heating Curve Of Naphthalene Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly. Heating Curve Of Naphthalene.
From www.researchgate.net
Naphthalene combustion vs. temperature for RhCoCliMor and RhCoCli Heating Curve Of Naphthalene The experiment described above can be summarized in a graph called a heating curve (figure below). The particles are very closely. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The graph above shows the heating curve of naphthalene. The electricity from the surroundings. Heating Curve Of Naphthalene.
From quizizz.com
Melting and boiling point of naphthalene Quizizz Heating Curve Of Naphthalene The graph above shows the heating curve of naphthalene. The particles are very closely. The experiment described above can be summarized in a graph called a heating curve (figure below). Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The sample is initially ice. Heating Curve Of Naphthalene.
From www.numerade.com
SOLVED Part 1 Graph Cooling Curve of Napthalene 1 1.00 10 Q6 Time Heating Curve Of Naphthalene The graph above shows the heating curve of naphthalene. The sample is initially ice at 1 atm. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. Once all the liquid has completely boiled away, continued heating of. Heating Curve Of Naphthalene.
From www.slideshare.net
Chapter 2 heating and cooling of naphthalene PPT Heating Curve Of Naphthalene The experiment described above can be summarized in a graph called a heating curve (figure below). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The particles are very closely. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the. Heating Curve Of Naphthalene.
From www.researchgate.net
TGA curves of naphthalene and mixture of HA/PMMA/naphthalene. Heating Heating Curve Of Naphthalene Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The graph above shows the heating curve of naphthalene. The sample is initially ice at 1 atm. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above. Heating Curve Of Naphthalene.
From www.slideshare.net
Chapter 2 heating and cooling of naphthalene Heating Curve Of Naphthalene The experiment described above can be summarized in a graph called a heating curve (figure below). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The graph above shows the heating curve of naphthalene. The particles are very closely. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time,. Heating Curve Of Naphthalene.
From www.slideserve.com
PPT Pretest PowerPoint Presentation, free download ID7091494 Heating Curve Of Naphthalene Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above. Heating Curve Of Naphthalene.
From www.knowledgeboat.com
The melting point of naphthalene is 80°C and the room KnowledgeBoat Heating Curve Of Naphthalene The experiment described above can be summarized in a graph called a heating curve (figure below). Naphthalene is in solid state at any temperature below its melting point. The graph above shows the heating curve of naphthalene. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The sample is initially ice at. Heating Curve Of Naphthalene.
From www.slideshare.net
Chapter 2 the structure of the atom Heating Curve Of Naphthalene The graph above shows the heating curve of naphthalene. The experiment described above can be summarized in a graph called a heating curve (figure below). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly. Heating Curve Of Naphthalene.
From www.doubtnut.com
melting point of naphthalene. Heating Curve Of Naphthalene The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1 atm.. Heating Curve Of Naphthalene.
From www.researchgate.net
(a) Heat capacity (C p ) for a molecular crystal of naphthalene from Heating Curve Of Naphthalene Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. Naphthalene is in solid state at any temperature below its melting. Heating Curve Of Naphthalene.
From question.pandai.org
Matter and the Atomic Structure Heating Curve Of Naphthalene The sample is initially ice at 1 atm. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The graph above shows the heating curve of naphthalene. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly. Heating Curve Of Naphthalene.
From byjus.com
5. The heat of combustion of naphthalene at constant volume at 25 Heating Curve Of Naphthalene Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1 atm. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas.. Heating Curve Of Naphthalene.
From www.pearson.com
Based on the phase diagram of CO2 shown in Figure 11.39(b), descr Heating Curve Of Naphthalene The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Figure \(\pageindex{3}\) shows. Heating Curve Of Naphthalene.
From www.youtube.com
Heating curve of Naphthalene YouTube Heating Curve Of Naphthalene Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. The graph above shows the heating curve of. Heating Curve Of Naphthalene.
From www.showme.com
Heat curve of naphthalene lab Science, Chemistry ShowMe Heating Curve Of Naphthalene The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The graph above. Heating Curve Of Naphthalene.
From www.scribd.com
Heating of Naphthalene Chemical Substances Water Heating Curve Of Naphthalene Naphthalene is in solid state at any temperature below its melting point. The graph above shows the heating curve of naphthalene. The sample is initially ice at 1 atm. The experiment described above can be summarized in a graph called a heating curve (figure below). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a. Heating Curve Of Naphthalene.
From www.youtube.com
Explanation of cooling curve of naphthalene YouTube Heating Curve Of Naphthalene Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts. Heating Curve Of Naphthalene.
From chemtribe.com
Heating and Cooling Curves ChemTribe Heating Curve Of Naphthalene Naphthalene is in solid state at any temperature below its melting point. The particles are very closely. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. The graph above shows the heating curve of naphthalene. The sample. Heating Curve Of Naphthalene.
From webapi.bu.edu
💋 Freezing point of naphthalene graph. What is the Hypothesis of the Heating Curve Of Naphthalene The experiment described above can be summarized in a graph called a heating curve (figure below). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The particles are very closely. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The electricity. Heating Curve Of Naphthalene.
From brainly.in
The melting point of naphthalene, a crystalline solid, is 80°C and the Heating Curve Of Naphthalene Naphthalene is in solid state at any temperature below its melting point. The sample is initially ice at 1 atm. The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. The graph above shows the heating curve of. Heating Curve Of Naphthalene.
From www.toppr.com
The melting point of naphthalene is 80°C and the room temperature is 30 Heating Curve Of Naphthalene Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The graph above shows the heating curve of naphthalene. The particles are very closely. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The. Heating Curve Of Naphthalene.
From www.researchgate.net
TGA curves of naphthalene and mixture of HA/PMMA/naphthalene. Heating Heating Curve Of Naphthalene The particles are very closely. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. Naphthalene is in solid state at any temperature below its melting point. The electricity from the surroundings (work). Heating Curve Of Naphthalene.
From www.researchgate.net
DSC second heating scan curves of naphthalenecontaining PI films with Heating Curve Of Naphthalene Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The particles are very closely. The electricity from the surroundings (work) heats the wire to a high temperature,. Heating Curve Of Naphthalene.
From www.slideshare.net
Chapter 2 heating and cooling of naphthalene Heating Curve Of Naphthalene The electricity from the surroundings (work) heats the wire to a high temperature, and heats the system slightly before it and the sample explode in the nearly 40 atm of o2 gas. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus. Heating Curve Of Naphthalene.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 1 SPM Chemistry Heating Curve Of Naphthalene Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The particles are very closely. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The experiment described above can be summarized in a graph. Heating Curve Of Naphthalene.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Heating Curve Of Naphthalene The sample is initially ice at 1 atm. Heating curve of naphthalene when naphthalene is heated, the temperature rises steadily until it starts to melt. The particles are very closely. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The electricity from the surroundings. Heating Curve Of Naphthalene.
From www.slideshare.net
Notechapter2 Heating Curve Of Naphthalene The experiment described above can be summarized in a graph called a heating curve (figure below). The sample is initially ice at 1 atm. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus. Heating Curve Of Naphthalene.
From mavink.com
Cooling Curve Of Naphthalene Heating Curve Of Naphthalene The experiment described above can be summarized in a graph called a heating curve (figure below). The particles are very closely. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The graph above shows the heating curve of naphthalene. Heating curve of naphthalene when naphthalene is heated, the temperature. Heating Curve Of Naphthalene.