How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 . How much iron can be recovered from 25 grams of f e x 2 o x 3 \ce{fe2o3} fe x 2 o x 3. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. To determine the amount of iron that can. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: If the fe2o3 is 99% pure, then. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. Oxygen gas can be produced by the decomposition of k c l o x 3. To know more about molar. The reaction will involve the use of an acid and a base.
from www.slideserve.com
To know more about molar. To determine the amount of iron that can. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. Oxygen gas can be produced by the decomposition of k c l o x 3. The reaction will involve the use of an acid and a base.
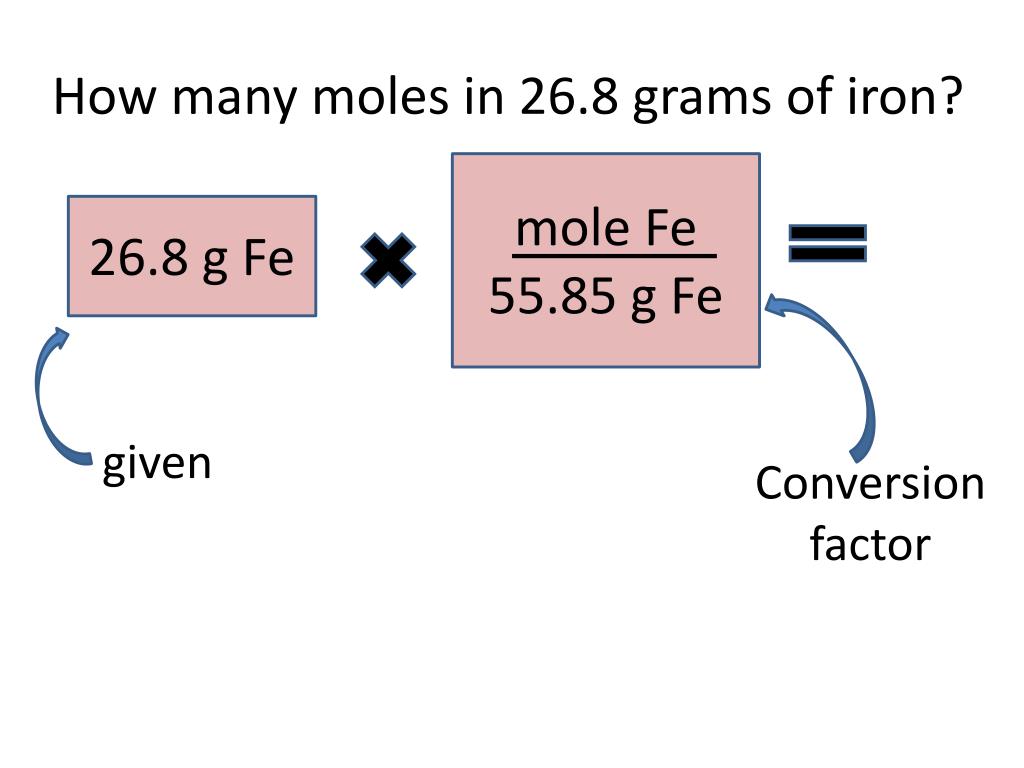
PPT 1 mole = 6.02 X 10 23 things This is called Avogadro’s number
How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To determine the amount of iron that can. How much iron can be recovered from 25 grams of f e x 2 o x 3 \ce{fe2o3} fe x 2 o x 3. To know more about molar. If the fe2o3 is 99% pure, then. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. The reaction will involve the use of an acid and a base. To determine the amount of iron that can. To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: Oxygen gas can be produced by the decomposition of k c l o x 3. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide.
From www.numerade.com
SOLVED Under certain conditions, the substances iron and oxygen How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. To know more about molar. To solve this question, we simply use the following formula to. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED What is the molarity of a solution that contains 10.0 grams of How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 If the fe2o3 is 99% pure, then. Oxygen gas can be produced by the decomposition of k c l o x 3. To know more about molar. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. To solve this question, we simply use the following formula to find the. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED Consider the following reaction 4AL 302 2Al203 What is the How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. Therefore, the main answer to this question is that 15 grams of iron. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. Oxygen gas can be produced by the decomposition of k c l o x 3. If. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From brainly.com
How many grams of Fe2O3 can form from 27.2 g of O2? 4Fe(s)+3O2(g How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. If the fe2o3 is 99% pure, then. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. Therefore, the main answer to this question is that. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 Oxygen gas can be produced by the decomposition of k c l o x 3. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. To solve this question, we simply use the following formula to find the. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.youtube.com
How to Convert Grams Fe to Moles (and Moles Fe to Atoms) YouTube How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The reaction will involve the use of an acid and a base. To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: To determine the amount of iron that can. Oxygen gas can be produced by the decomposition of k c l o x 3. How much iron can. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED Combining 0.232 mol Fe2O3 with excess carbon produced 14.1 g Fe How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. To determine the amount of iron that can. How much iron can be recovered from 25 grams of f. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From brainly.com
How many grams of Fe2O3 will be produced from 37.5 grams of iron How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To determine the amount of iron that can. To know more about molar. If the fe2o3 is 99% pure, then. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide.. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.slideserve.com
PPT 1 mole = 6.02 X 10 23 things This is called Avogadro’s number How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To know more about molar. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: The amount of iron that can be recovered from 25.0 g of fe₂o₃ is. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVEDConsider the following equation C3 H8(g)+5 O2(g) 3 CO2(g)+4 H2 How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The reaction will involve the use of an acid and a base. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. If the fe2o3 is 99% pure, then. How much iron can be recovered. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED The equation FezO3 3C = 2Fe 3CO, tells us that mole of iron How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. The reaction will involve. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED Using the following equation Fe2O3(s) + 3H2(g), how many moles How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.coursehero.com
[Solved] How many grams of O2 are needed to produce 45.8 grams of Fe2O3 How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. To determine the amount of iron that can. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. Oxygen gas can be produced by the decomposition of k. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED How many grams of iron(III) sulfide form when 62.0 mL of 0.135 How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 Oxygen gas can be produced by the decomposition of k c l o x 3. How much iron can be recovered from 25 grams of f e x 2 o x 3 \ce{fe2o3} fe x 2 o x 3. The reaction will involve the use of an acid and a base. To determine the amount of iron that can. Therefore,. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.toppr.com
How many grams of potassium chloride (KCl) must be added to make 500 mL How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To know more about molar. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From capitalizemytitle.com
How Many Grams in an Ounce (oz to g)? Conversions & Calculator How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 If the fe2o3 is 99% pure, then. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. To determine the amount of iron that can. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. Oxygen gas can be produced by the decomposition. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From brainly.com
1. How many grams of iron (II) oxide can be produced from 3.4 g of iron How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. To determine the amount of iron that can. To know more about molar. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. If the fe2o3 is 99% pure, then. To solve this question, we. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From brainly.com
aluminum reacts with 42g of iron(ii) sulfate. How many grams of iron How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. The reaction will involve the use of an acid and a base. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. If. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From brainly.com
Please help! How many grams of Fe2O3 of are needed to react with an How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 How much iron can be recovered from 25 grams of f e x 2 o x 3 \ce{fe2o3} fe x 2 o x 3. Oxygen gas can be produced by the decomposition of k c l o x 3. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. To know more about molar. To. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.youtube.com
How many grams of hydrogen gas are needed to produce 5.00 g of water How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 If the fe2o3 is 99% pure, then. The reaction will involve the use of an acid and a base. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. Therefore, the main answer to this. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.coursehero.com
[Solved] Fe2O3+3H2 > 2Fe+3H2O a) how many grams of iron are made from How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. To determine the amount of iron that can. The iron can be recovered. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.toppr.com
How much of iron can be theoretically obtained by the reduction of 1 kg How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To know more about molar. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. To solve this question, we simply use the following formula to find the mass, m (fe), of iron. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVEDHow many grams of water are required to dissolve 12.7 grams of How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. To know more about molar. The reaction will involve the use of an acid and a base. If the fe2o3 is 99% pure, then. Therefore, the main answer to this question is that. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.chegg.com
Solved 4Fe + 3O2 → 2Fe2O3 If 36 grams of iron react with 22 How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: To determine the amount of iron that can. Oxygen gas can be produced by the decomposition of k c l o x 3. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.pdfprof.com
Section 1. Titre de la procédure Résolution pacifique des différends How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The reaction will involve the use of an acid and a base. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. To determine the amount of iron that can. To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: How much iron can. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.chegg.com
Solved 5. Calculate how many grams of iron can be made from How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. If the fe2o3 is 99% pure, then. Oxygen gas can be produced by the decomposition of k c l o x 3. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The reaction will involve the use of an acid and a base. If the fe2o3 is 99% pure, then. To determine the amount of iron that can. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. To solve this question, we simply. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED How many grams of O2 are needed to produce 45.8 grams of Fe2O3 How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. How much iron can be recovered from 25 grams of f e x 2 o x 3 \ce{fe2o3}. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVEDHow many grams of NH3 could be made using 6 moles of H2 and How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 Oxygen gas can be produced by the decomposition of k c l o x 3. To know more about molar. To determine the amount of iron that can. The amount of iron that can be extracted from 25.0g of fe2o3 will depend on the purity of the oxide. The amount of iron that can be recovered from 25.0 g of. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED Given the following reaction Fe2O3(s) + CO(g) 🡺 Fe(s) + CO2(g How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. Oxygen gas can be produced by the decomposition of k c l o x 3. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. If the fe2o3 is 99% pure, then. The. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.numerade.com
SOLVED Iron pyrite (FeS2) reacts with oxygen according to the How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 To solve this question, we simply use the following formula to find the mass, m (fe), of iron we can recover: The reaction will involve the use of an acid and a base. To determine the amount of iron that can. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From solvedlib.com
How many grams of iron (ii) iodide contain 4.60 grams… SolvedLib How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 Oxygen gas can be produced by the decomposition of k c l o x 3. Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of fe2o3. To determine the amount of iron that can. How much iron can be recovered from 25 grams of f e x 2 o x 3. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From exymxlkfq.blob.core.windows.net
How Many Grams Are In 1 Mole Of Iron at Gary Rencher blog How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 If the fe2o3 is 99% pure, then. The amount of iron that can be recovered from 25.0 g of fe₂o₃ is approximately 17.42 g. The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. To determine the amount of iron that can. Therefore, the main answer to this question is that 15 grams of iron. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.
From www.coursehero.com
[Solved] Determine the mass in grams of CO2 that is produced by the How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3 The iron can be recovered from 25 grams of fe2o3 by using a chemical reaction. To determine the amount of iron that can. If the fe2o3 is 99% pure, then. How many grams of iron can be recovered from 25.0 g of fe_(2)o_(3) ask ai pdf chat calculator subjects feedback upgrade to plus log in home /. How much iron. How Many Grams Of Iron Can Be Recovered From 25 0G Of Fe2O3.