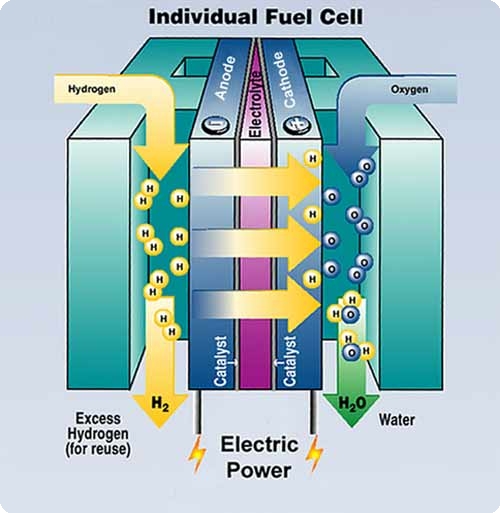
Fuel Cells Engineering Chemistry . Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Fuel cells are similar to batteries but. A fuel cell resembles a battery in. Thermodynamics of the fuel cell. Irreversible losses in fuel cell. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. A fuel cell is a device that converts chemical energy into electrical energy. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Introduction and overview of fuel cell.
from exohobyin.blob.core.windows.net
A fuel cell is a device that converts chemical energy into electrical energy. Introduction and overview of fuel cell. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Thermodynamics of the fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Irreversible losses in fuel cell. Fuel cells are similar to batteries but. A fuel cell resembles a battery in.
Fuel Cell Engineering Chemistry at Carole Jackson blog
Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. A fuel cell resembles a battery in. Fuel cells are similar to batteries but. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. A fuel cell is a device that converts chemical energy into electrical energy. Introduction and overview of fuel cell. Thermodynamics of the fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Irreversible losses in fuel cell.
From iupac.org
Fuel Cells IUPAC International Union of Pure and Applied Chemistry Fuel Cells Engineering Chemistry Fuel cells are similar to batteries but. A fuel cell resembles a battery in. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Introduction and overview of fuel cell. Thermodynamics of the fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert. Fuel Cells Engineering Chemistry.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cells Engineering Chemistry Fuel cells are similar to batteries but. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Thermodynamics of the fuel cell. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. A fuel cell resembles a battery in. Fuel cell technology offers the opportunity of. Fuel Cells Engineering Chemistry.
From www.semanticscholar.org
Figure I from Development in Direct Methanol Oxygen Fuel Cell (DMFC Fuel Cells Engineering Chemistry A fuel cell resembles a battery in. Irreversible losses in fuel cell. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Introduction and overview of fuel cell. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run. Fuel Cells Engineering Chemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Introduction and overview of fuel cell. Irreversible losses in fuel cell. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of. Fuel Cells Engineering Chemistry.
From www.nafion.com
Innovative Fuel Cells Enabled by Ion Exchange Membranes Fuel Cells Engineering Chemistry Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Introduction and overview of fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Thermodynamics of the fuel cell. A fuel cell resembles a battery in. The. Fuel Cells Engineering Chemistry.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cells Engineering Chemistry A fuel cell is a device that converts chemical energy into electrical energy. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Thermodynamics of the fuel cell. A fuel cell resembles a battery in. Irreversible losses in fuel cell. Fuel cells are similar to batteries but. Fuel cell technology offers the opportunity. Fuel Cells Engineering Chemistry.
From www.engineeringa2z.com
Fuel Cell Construction and Working Engineeringa2z Fuel Cells Engineering Chemistry Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Introduction and overview of fuel cell. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Fuel cells are similar to batteries but. Fuel cell, any of a class. Fuel Cells Engineering Chemistry.
From studylib.net
Fuel Cell Engineering Fuel Cells Engineering Chemistry Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Introduction and overview of fuel cell. A fuel cell resembles a battery in. Thermodynamics of the fuel cell. A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are electrochemical membrane. Fuel Cells Engineering Chemistry.
From www.dreamstime.com
Fuel Cell and Liion Battery Diagram. Vector. Device that Converts Fuel Cells Engineering Chemistry Introduction and overview of fuel cell. A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Thermodynamics of. Fuel Cells Engineering Chemistry.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Fuel Cells Engineering Chemistry Fuel cells are similar to batteries but. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Introduction and overview of fuel cell. Irreversible losses in fuel cell. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. A. Fuel Cells Engineering Chemistry.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Fuel Cells Engineering Chemistry Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Irreversible losses in fuel cell. Fuel cell, any of a class of devices that convert the chemical energy of. Fuel Cells Engineering Chemistry.
From www.mdpi.com
Energies Free FullText Optimal Parameter Identification of a PEM Fuel Cells Engineering Chemistry Thermodynamics of the fuel cell. Introduction and overview of fuel cell. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. A fuel cell is a device that converts chemical energy. Fuel Cells Engineering Chemistry.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Thermodynamics of the fuel cell. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Fuel cell, any of a class of devices that convert the chemical energy of. Fuel Cells Engineering Chemistry.
From pubs.acs.org
Comprehensive Review of FuelCellType Sensors for Gas Detection Fuel Cells Engineering Chemistry Irreversible losses in fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Thermodynamics of the fuel cell. A fuel cell resembles a battery in. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell technology offers the opportunity of creating environmentally. Fuel Cells Engineering Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Fuel Cells Engineering Chemistry Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Introduction and overview of fuel cell. Irreversible losses in fuel cell. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is a device that converts chemical energy. Fuel Cells Engineering Chemistry.
From exohobyin.blob.core.windows.net
Fuel Cell Engineering Chemistry at Carole Jackson blog Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Thermodynamics of the fuel cell. Fuel cell, any of a class of devices that convert the chemical energy of. Fuel Cells Engineering Chemistry.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Introduction and overview of fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Fuel cells are similar to batteries but. A fuel cell is a device that converts chemical energy into electrical energy.. Fuel Cells Engineering Chemistry.
From www.researchgate.net
Scheme of a solid oxide fuel cell. Download Scientific Diagram Fuel Cells Engineering Chemistry A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are similar to batteries but. Introduction and overview of fuel cell. Thermodynamics of the fuel cell. Irreversible losses in fuel cell. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. A fuel cell resembles a battery in.. Fuel Cells Engineering Chemistry.
From studylib.net
Fuel Cell Efficiency Chemical Engineering Fuel Cells Engineering Chemistry Introduction and overview of fuel cell. Fuel cells are similar to batteries but. A fuel cell resembles a battery in. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy. Fuel Cells Engineering Chemistry.
From pressbooks.bccampus.ca
5.6 Batteries and Fuel Cells Chemistry for Chemical Engineers Fuel Cells Engineering Chemistry Thermodynamics of the fuel cell. Introduction and overview of fuel cell. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Irreversible losses in fuel cell. Fuel cell, any. Fuel Cells Engineering Chemistry.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Fuel Cells Engineering Chemistry Irreversible losses in fuel cell. Fuel cells are similar to batteries but. A fuel cell resembles a battery in. A fuel cell is a device that converts chemical energy into electrical energy. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell technology offers the opportunity of creating environmentally friendly portable. Fuel Cells Engineering Chemistry.
From www.youtube.com
Fuel Cell O level / IGCSE Chemistry YouTube Fuel Cells Engineering Chemistry A fuel cell is a device that converts chemical energy into electrical energy. Irreversible losses in fuel cell. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. A fuel cell resembles a battery in. Fuel. Fuel Cells Engineering Chemistry.
From www.youtube.com
METHANOL OXYGEN FUEL CELL FUEL CELLS Engineering Chemistry Fuel Cells Engineering Chemistry Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell resembles a battery in. Irreversible losses in fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored. Fuel Cells Engineering Chemistry.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Fuel Cells Engineering Chemistry A fuel cell resembles a battery in. A fuel cell is a device that converts chemical energy into electrical energy. Irreversible losses in fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy. Fuel Cells Engineering Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. A fuel cell is a device that converts chemical energy into electrical energy. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Introduction and overview of fuel cell.. Fuel Cells Engineering Chemistry.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cells Engineering Chemistry Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. The advantages, problems and applications associated with each of the different electrolytes commonly used. Fuel Cells Engineering Chemistry.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Fuel Cells Engineering Chemistry Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Introduction and overview of fuel cell. Fuel cell technology offers the opportunity of creating. Fuel Cells Engineering Chemistry.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. A fuel cell resembles a battery in. Fuel cells are similar to batteries but. A fuel cell is a. Fuel Cells Engineering Chemistry.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Fuel Cells Engineering Chemistry A fuel cell is a device that converts chemical energy into electrical energy. Introduction and overview of fuel cell. Thermodynamics of the fuel cell. Irreversible losses in fuel cell. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are electrochemical membrane reactors that are able. Fuel Cells Engineering Chemistry.
From www.youtube.com
Engineering Chemistry Module III Fuel Cells YouTube Fuel Cells Engineering Chemistry A fuel cell resembles a battery in. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. Irreversible losses in fuel cell. A fuel cell is a device that. Fuel Cells Engineering Chemistry.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cells Engineering Chemistry A fuel cell is a device that converts chemical energy into electrical energy. Introduction and overview of fuel cell. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles.. Fuel Cells Engineering Chemistry.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cells Engineering Chemistry Introduction and overview of fuel cell. A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell resembles a battery in. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly. Fuel Cells Engineering Chemistry.
From fuelcellsworks.com
University Of Delaware Engineers Develop Fuel Cell System That Helps Fuel Cells Engineering Chemistry Irreversible losses in fuel cell. Fuel cells are similar to batteries but. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Thermodynamics of the fuel cell. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices. Fuel Cells Engineering Chemistry.
From www.dataphysics-instruments.com
How measuring contact angles at high temperatures can help develop fuel Fuel Cells Engineering Chemistry Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Irreversible losses in fuel cell. Introduction and overview of fuel cell. Fuel cells are similar to batteries but. Fuel. Fuel Cells Engineering Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Fuel Cells Engineering Chemistry The advantages, problems and applications associated with each of the different electrolytes commonly used in fuel cells,. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to electrical. Fuel cells are similar. Fuel Cells Engineering Chemistry.