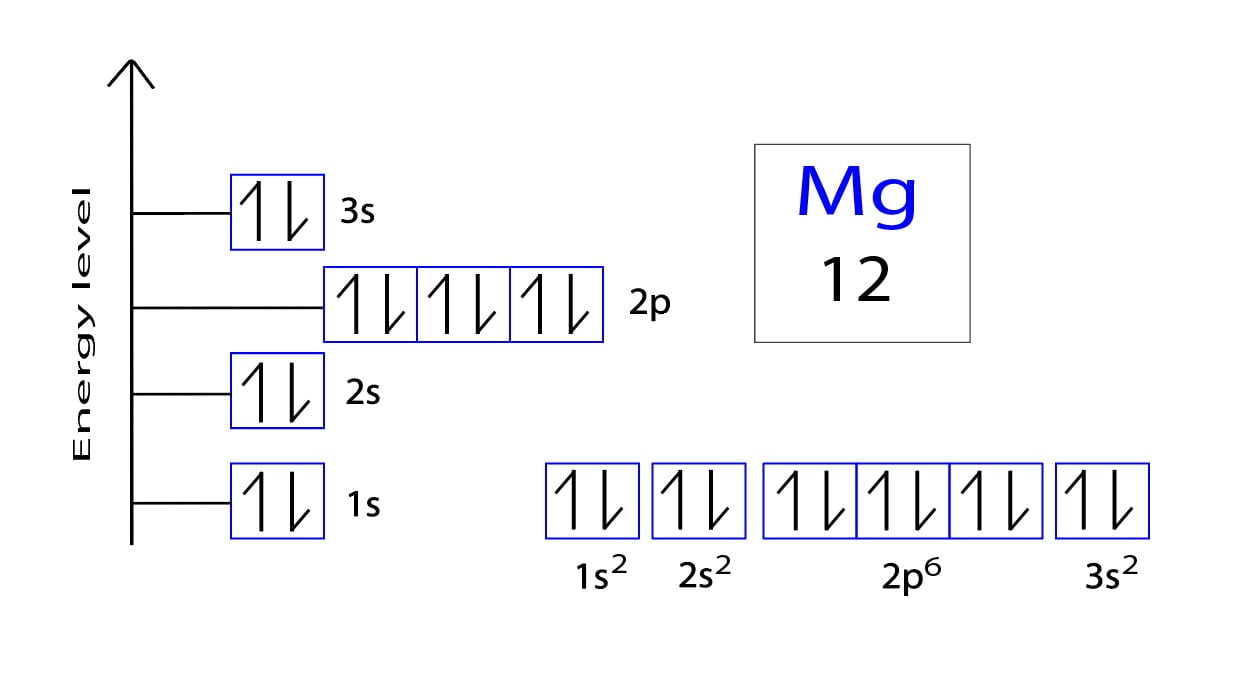
Magnesium Electron Configuration Class 9 . This notation shows the distribution of electrons across shells and. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The electron configuration of an atom is written with the help of subshell labels. (a) give the electron configuration of magnesium and chlorine. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: View rotating bohr models for all 118. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements:
from valenceelectrons.com
This notation shows the distribution of electrons across shells and. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. The electron configuration of an atom is written with the help of subshell labels. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). These labels contain the shell number (given by the principal quantum number), the subshell name (given by. (a) give the electron configuration of magnesium and chlorine. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements:
Electron Configuration for Magnesium(Mg, Mg2+ ion)
Magnesium Electron Configuration Class 9 The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. This notation shows the distribution of electrons across shells and. View rotating bohr models for all 118. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: These labels contain the shell number (given by the principal quantum number), the subshell name (given by. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Access detailed info on all elements: (a) give the electron configuration of magnesium and chlorine. Atomic mass, electron configurations, charges, and more. The electron configuration of an atom is written with the help of subshell labels. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium Electron Configuration Class 9 (a) give the electron configuration of magnesium and chlorine. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. This notation shows the distribution of electrons across shells and. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. The electron. Magnesium Electron Configuration Class 9.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Configuration Class 9 (a) give the electron configuration of magnesium and chlorine. This notation shows the distribution of electrons across shells and. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: In order to write the mg electron configuration we first need to know. Magnesium Electron Configuration Class 9.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Configuration Class 9 Atomic mass, electron configurations, charges, and more. (a) give the electron configuration of magnesium and chlorine. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). This notation shows the distribution of electrons across shells and. The complete electron configuration for magnesium should be written. Magnesium Electron Configuration Class 9.
From charlee-yersblogbarnes.blogspot.com
Atomic Mass of Magnesium Magnesium Electron Configuration Class 9 Access detailed info on all elements: The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: (a) give the electron configuration of magnesium and chlorine. The electron configuration of an atom is. Magnesium Electron Configuration Class 9.
From www.numerade.com
SOLVED Write the complete electron configuration for the magnesium Magnesium Electron Configuration Class 9 Access detailed info on all elements: Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: This notation shows the distribution of electrons across shells and. Atomic mass, electron configurations, charges, and more. (a) give the electron configuration of magnesium and chlorine. View rotating bohr models for all 118. These labels contain the shell. Magnesium Electron Configuration Class 9.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Electron Configuration Class 9 The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. (a) give the electron configuration of magnesium and chlorine. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. Access detailed info on all elements: This notation shows the distribution of electrons across shells and. Chapter 4 of ncert class 9. Magnesium Electron Configuration Class 9.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Configuration Class 9 In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. View. Magnesium Electron Configuration Class 9.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Electron Configuration Class 9 Access detailed info on all elements: (a) give the electron configuration of magnesium and chlorine. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. In order to write the mg. Magnesium Electron Configuration Class 9.
From www.coursehero.com
[Solved] Write the short form electron configuration for magnesium Magnesium Electron Configuration Class 9 The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. This notation shows the distribution of electrons across shells and. Atomic mass, electron configurations, charges, and more. Access detailed info on. Magnesium Electron Configuration Class 9.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Electron Configuration Class 9 In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). (a) give the electron configuration of magnesium and chlorine. Access detailed info on all elements: The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is. Magnesium Electron Configuration Class 9.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Configuration Class 9 The electron configuration of an atom is written with the help of subshell labels. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. Access detailed info on all elements: Atomic mass,. Magnesium Electron Configuration Class 9.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Electron Configuration Class 9 Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). (a) give the electron configuration of magnesium and chlorine. The electron configuration of an atom is written with the. Magnesium Electron Configuration Class 9.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Magnesium Electron Configuration Class 9 These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The electron configuration of an atom is written with the help of subshell labels. View rotating bohr models for all 118. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. In order to write the mg electron configuration we first. Magnesium Electron Configuration Class 9.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Class 9 The electron configuration of an atom is written with the help of subshell labels. (a) give the electron configuration of magnesium and chlorine. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118. This notation shows the distribution of electrons across shells and. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Access. Magnesium Electron Configuration Class 9.
From www.youtube.com
Magnesium electronic configuration How to Write Magnesium electronic Magnesium Electron Configuration Class 9 These labels contain the shell number (given by the principal quantum number), the subshell name (given by. Access detailed info on all elements: The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. This notation shows the distribution of electrons across shells and. View rotating bohr models for all 118. The complete electron configuration for magnesium should be. Magnesium Electron Configuration Class 9.
From trueufiles204.weebly.com
Magnesium Valence Electrons trueufiles Magnesium Electron Configuration Class 9 The electron configuration of an atom is written with the help of subshell labels. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. This notation shows the distribution of electrons across shells and. Atomic mass,. Magnesium Electron Configuration Class 9.
From www.chegg.com
Solved Electronic configuration of Magnesium is Select one Magnesium Electron Configuration Class 9 In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of an atom is written with the help of subshell labels. Access detailed info on all elements: This notation shows the distribution of electrons across shells and. The complete electron configuration for. Magnesium Electron Configuration Class 9.
From www.youtube.com
Valency of magnesium CBSE Class 9 Chemistry notes YouTube Magnesium Electron Configuration Class 9 Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: This notation shows the distribution of electrons across shells and. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. The electron configuration of an atom is written with the help of. Magnesium Electron Configuration Class 9.
From www.toppr.com
Write the electron dot structure of magnesium and oxygen. Magnesium Electron Configuration Class 9 The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. This notation shows the distribution of electrons across shells and. View rotating bohr models for all 118. Access detailed info on all elements: Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: The electron configuration of an atom is written with. Magnesium Electron Configuration Class 9.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Class 9 The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. This notation shows the distribution of electrons across shells and. (a) give the electron configuration of magnesium and chlorine. Atomic mass, electron configurations, charges, and more. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: Access detailed info on all elements:. Magnesium Electron Configuration Class 9.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Configuration Class 9 In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). These labels contain the shell number (given by the principal quantum number), the subshell name (given by. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: The complete. Magnesium Electron Configuration Class 9.
From guidemanualeruptivity.z14.web.core.windows.net
Electronic Structure Diagram For Magnesium Magnesium Electron Configuration Class 9 (a) give the electron configuration of magnesium and chlorine. View rotating bohr models for all 118. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The electron configuration of an atom is written with the help of subshell labels. In. Magnesium Electron Configuration Class 9.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Electron Configuration Class 9 (a) give the electron configuration of magnesium and chlorine. Atomic mass, electron configurations, charges, and more. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Access detailed info on all elements: View rotating bohr models for all 118. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: The electron configuration. Magnesium Electron Configuration Class 9.
From www.shutterstock.com
Diagram Representation Of The Element Magnesium Illustration Magnesium Electron Configuration Class 9 This notation shows the distribution of electrons across shells and. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. Access detailed info on all elements: In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron. Magnesium Electron Configuration Class 9.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Magnesium Electron Configuration Class 9 Atomic mass, electron configurations, charges, and more. This notation shows the distribution of electrons across shells and. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Access detailed info on all elements: The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. (a) give the. Magnesium Electron Configuration Class 9.
From ar.inspiredpencil.com
Magnesium Atom Diagram Magnesium Electron Configuration Class 9 These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: The electron configuration of an atom is written with the help of subshell labels. View. Magnesium Electron Configuration Class 9.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Electron Configuration Class 9 Atomic mass, electron configurations, charges, and more. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. View rotating bohr models for all 118. The electron configuration of an atom is written with the help of subshell labels. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is. Magnesium Electron Configuration Class 9.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Class 9 These labels contain the shell number (given by the principal quantum number), the subshell name (given by. View rotating bohr models for all 118. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. This notation shows the distribution of electrons across shells and. In order to write. Magnesium Electron Configuration Class 9.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Magnesium Electron Configuration Class 9 This notation shows the distribution of electrons across shells and. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118. (a) give the electron configuration of magnesium and chlorine. Access detailed info on all. Magnesium Electron Configuration Class 9.
From brainly.com
What is the electron configuration for the Magnesium ion? Magnesium Electron Configuration Class 9 Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: This notation shows the distribution of electrons across shells and. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. The electron configuration of an atom is written with the help of. Magnesium Electron Configuration Class 9.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Class 9 View rotating bohr models for all 118. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. The electron configuration of an atom is written with the help of subshell labels.. Magnesium Electron Configuration Class 9.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Configuration Class 9 Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: These labels contain the shell number (given by the principal quantum number), the subshell name (given by. (a) give the electron configuration of magnesium and chlorine. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. The complete electron configuration for magnesium. Magnesium Electron Configuration Class 9.
From ar.inspiredpencil.com
Electron Configuration Magnesium Magnesium Electron Configuration Class 9 (a) give the electron configuration of magnesium and chlorine. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. View rotating bohr models for all 118. The complete electron configuration for magnesium should be written as 1s2 2s2 2p6 3s2 and the unabbreviated electron configuration is [ne] 3s2. These labels contain the shell number (given by the principal. Magnesium Electron Configuration Class 9.
From truebfil778.weebly.com
Number Of Electrons In Magnesium truebfil Magnesium Electron Configuration Class 9 This notation shows the distribution of electrons across shells and. The electron configuration of magnesium is written as 1s2 2s2 2p6 3s2. These labels contain the shell number (given by the principal quantum number), the subshell name (given by. Access detailed info on all elements: View rotating bohr models for all 118. Chapter 4 of ncert class 9 science discusses. Magnesium Electron Configuration Class 9.
From thefitnessmanual.com
Electronic Configuration Of Magnesium Class 9 TheFitnessManual Magnesium Electron Configuration Class 9 These labels contain the shell number (given by the principal quantum number), the subshell name (given by. The electron configuration of an atom is written with the help of subshell labels. Chapter 4 of ncert class 9 science discusses the structure of atoms, focusing on subatomic particles: This notation shows the distribution of electrons across shells and. (a) give the. Magnesium Electron Configuration Class 9.