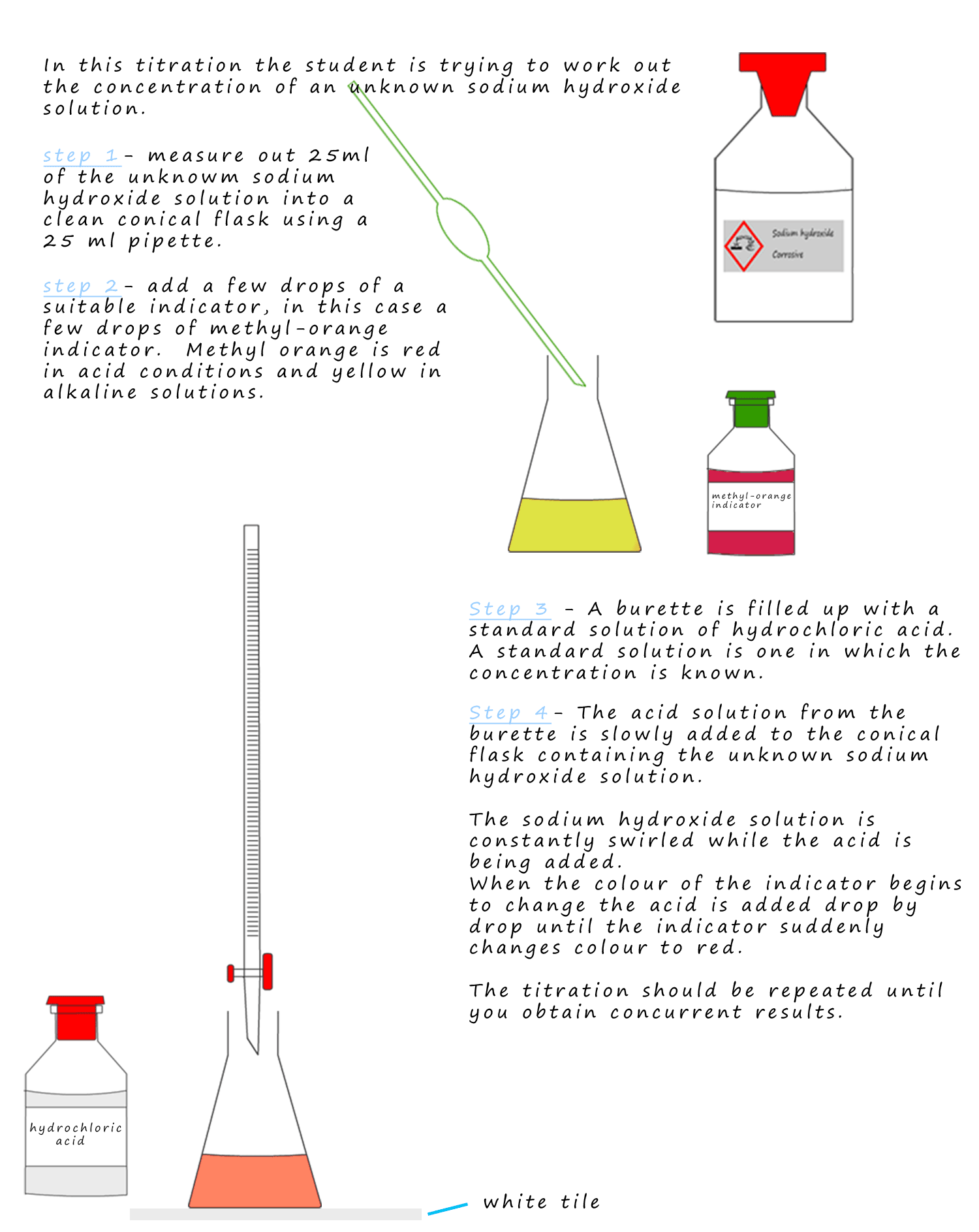
Titration Yellow Color . 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. Indicators are substances whose solutions change color due to changes in ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow.
from www.science-revision.co.uk
When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. Indicators are substances whose solutions change color due to changes in ph. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it.
Titrations
Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Indicators are substances whose solutions change color due to changes in ph. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow.
From slideplayer.com
Definitions and Properties ppt download Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. Indicators are substances whose solutions change color due to changes in ph.. Titration Yellow Color.
From www.chegg.com
Solved (15) Determine the color of the solution based on the Titration Yellow Color The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. Indicators are substances whose solutions change color due to changes in ph. If you. Titration Yellow Color.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. 66 rows the audience of users, the decision whether to determine. Titration Yellow Color.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest Titration Yellow Color Indicators are substances whose solutions change color due to changes in ph. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. When. Titration Yellow Color.
From www.science-revision.co.uk
Titrations Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. If you use methyl orange, you expect the color to change. Titration Yellow Color.
From slideplayer.com
Titration Colour Changes ppt download Titration Yellow Color Indicators are substances whose solutions change color due to changes in ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from. Titration Yellow Color.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Indicators are substances whose solutions change color due to changes in ph. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. When. Titration Yellow Color.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The litmus color change happens over an unusually wide range, but. Titration Yellow Color.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. The litmus color change happens over. Titration Yellow Color.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Yellow Color The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. If you use methyl orange, you expect the color to change. Titration Yellow Color.
From flatworldknowledge.lardbucket.org
Quantitative Analysis Using Titrations Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. Indicators are substances whose solutions change color due to changes in ph. The. Titration Yellow Color.
From stock.adobe.com
The titration process is done by dripping the titrant solution into the Titration Yellow Color 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Indicators are substances whose solutions change color due to changes in ph. The. Titration Yellow Color.
From mungfali.com
Acid Base Titration Indicator Titration Yellow Color 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. As in case of thymol blue, in very strong acidic solutions (ph < 1.8),. Titration Yellow Color.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Yellow Color The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. When 25 ml of titrant has been added (the equivalence point),. Titration Yellow Color.
From slidetodoc.com
Analytical Chemistry Neutral Titration Introduction n n Neutral Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. Indicators are substances whose solutions change color due to changes in ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear. Titration Yellow Color.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Yellow Color Indicators are substances whose solutions change color due to changes in ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion. Titration Yellow Color.
From chemistnotes.com
Non Aqueous Titration Types, indicators, solvents, advantages Titration Yellow Color The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. Indicators are substances whose solutions change color due to changes in ph. As in. Titration Yellow Color.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration Yellow Color If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. As in case of thymol blue, in very strong. Titration Yellow Color.
From www.vectorstock.com
Phenolphthalein indicator in acidbase titration Vector Image Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. When 25 ml of titrant has been added (the equivalence point), the. Titration Yellow Color.
From www.scribd.com
Titrations Yellow Group PDF Titration Yellow Color If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. When 25 ml of titrant has been. Titration Yellow Color.
From www.sciencephoto.com
End point of an iodine titration. 2 of 5. Stock Image C029/1115 Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. 66 rows the audience of users, the decision whether to determine. Titration Yellow Color.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Titration Yellow Color If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it. Indicators are substances whose solutions change color. Titration Yellow Color.
From www.mometrix.com
Titration Chemistry Review (Video) Titration Yellow Color If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. As in case of thymol blue,. Titration Yellow Color.
From mirjamglessmer.com
Winkler titration Archives Adventures in Oceanography and Teaching Titration Yellow Color If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Indicators are substances whose solutions change. Titration Yellow Color.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Titration Yellow Color Indicators are substances whose solutions change color due to changes in ph. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion. Titration Yellow Color.
From www.youtube.com
Color change in Titration YouTube Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. When 25 ml of titrant has been. Titration Yellow Color.
From www.chegg.com
Solved Color at Start of Titrationlight green, visibility Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Indicators are substances whose solutions change color due to changes in. Titration Yellow Color.
From mavink.com
Titration Color Titration Yellow Color Indicators are substances whose solutions change color due to changes in ph. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. The litmus color change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it.. Titration Yellow Color.
From cbceskillindia.com
Why does color change in titration? Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. If you use methyl orange, you expect the color to change from yellow to. Titration Yellow Color.
From ar.inspiredpencil.com
Titration Phenolphthalein Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. 66 rows the audience of users, the decision whether to determine. Titration Yellow Color.
From fphoto.photoshelter.com
science chemistry titration methyl red Fundamental Photographs The Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. 66 rows the audience of users, the decision whether to. Titration Yellow Color.
From ontaonta.com
OntaOnta Chemistry 11 Titration Yellow Color When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. As in case of thymol blue, in very strong acidic solutions (ph <. Titration Yellow Color.
From mavink.com
Titration Color Titration Yellow Color 66 rows the audience of users, the decision whether to determine a titration error for a particular color change and the experimental. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. If you use methyl orange, you expect the color to change from yellow. Titration Yellow Color.
From www.numerade.com
SOLVED Consider the titration of 50.0 mL of a weak diprotic acid, HzA Titration Yellow Color Indicators are substances whose solutions change color due to changes in ph. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated. Titration Yellow Color.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Yellow Color As in case of thymol blue, in very strong acidic solutions (ph < 1.8), the quinoide structure is protonated and the zwitter ion is. If you use methyl orange, you expect the color to change from yellow to orange somewhere below a ph of 6 and from orange to red around a ph. 66 rows the audience of users, the. Titration Yellow Color.