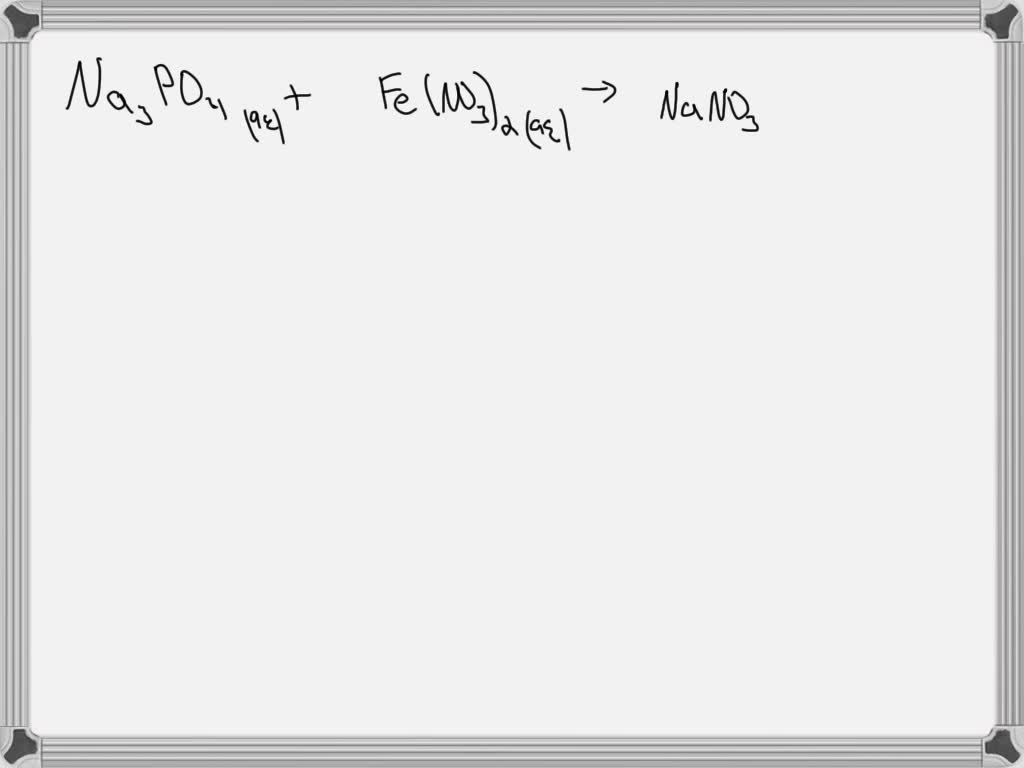Iron Copper Ii Nitrate Balanced Equation . The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. In the activity series, fe is more reactive than cu, so the reaction. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Enter an equation of an ionic chemical equation and press the balance button. See examples, instructions and tips. Consider the following sr reaction between iron metal and copper(ii) nitrate: Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. The balanced equation will be calculated along with the. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation.
from www.numerade.com
First, we need to check if fe will displace cu in the copper(ii) nitrate solution. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. The balanced equation will be calculated along with the. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Consider the following sr reaction between iron metal and copper(ii) nitrate: In the activity series, fe is more reactive than cu, so the reaction. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. Enter an equation of an ionic chemical equation and press the balance button. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and.
SOLVED Write the balanced molecular equation, including phases, for
Iron Copper Ii Nitrate Balanced Equation In the activity series, fe is more reactive than cu, so the reaction. In the activity series, fe is more reactive than cu, so the reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. Consider the following sr reaction between iron metal and copper(ii) nitrate: See examples, instructions and tips. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. Enter an equation of an ionic chemical equation and press the balance button. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. The balanced equation will be calculated along with the.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Iron Copper Ii Nitrate Balanced Equation In the activity series, fe is more reactive than cu, so the reaction. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver. Iron Copper Ii Nitrate Balanced Equation.
From www.guidechem.com
13476089 Iron(II) nitrate hexahydrate. Sale from Quality Iron Copper Ii Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. In the activity series, fe is more reactive than cu, so the reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate. Iron Copper Ii Nitrate Balanced Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Iron Copper Ii Nitrate Balanced Equation First, we need to check if fe will displace cu in the copper(ii) nitrate solution. Enter an equation of an ionic chemical equation and press the balance button. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Consider the following. Iron Copper Ii Nitrate Balanced Equation.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Iron Copper Ii Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. In this video we'll balance the equation cu(no3)2 = cuo + no2 +. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED balanced equation; Solutions of magnesium iodide and copper (II Iron Copper Ii Nitrate Balanced Equation In the activity series, fe is more reactive than cu, so the reaction. See examples, instructions and tips. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Enter a chemical equation. Iron Copper Ii Nitrate Balanced Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Iron Copper Ii Nitrate Balanced Equation In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. See examples, instructions and tips. In the activity series, fe is more reactive than cu, so the reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The balanced equation will. Iron Copper Ii Nitrate Balanced Equation.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Iron Copper Ii Nitrate Balanced Equation See examples, instructions and tips. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. In the activity series, fe is more reactive than cu, so the reaction. Iron metal is added to a solution of copper (ii) nitrate express your. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Give the complete ionic equation for the reaction (if any) that Iron Copper Ii Nitrate Balanced Equation Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. Identify the oxidation numbers, determine the changes. Iron Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] Suppose 0.0615g of copper(II) nitrate is dissolved in 50.mL of Iron Copper Ii Nitrate Balanced Equation Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce. Iron Copper Ii Nitrate Balanced Equation.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Iron Copper Ii Nitrate Balanced Equation Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The balanced equation will be calculated along with the. Consider the following sr reaction between iron. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper Iron Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Consider the following sr reaction between iron metal and copper(ii) nitrate: See examples, instructions and tips. In the activity series, fe is more reactive than cu, so. Iron Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved VI. Write formula equations and balance with Iron Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. In the activity series, fe is more reactive than cu, so the reaction. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. First, we need to check. Iron Copper Ii Nitrate Balanced Equation.
From www.toppr.com
21. Express the following reaction in the form of a balanced chemical Iron Copper Ii Nitrate Balanced Equation In the activity series, fe is more reactive than cu, so the reaction. See examples, instructions and tips. The balanced equation will be calculated along with the. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Enter an equation of an ionic chemical equation and press the balance button. Fe(s). Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
2. Copper Nitrate to Copper Hydroxide (Cu Again Lab) YouTube Iron Copper Ii Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. In this video we'll balance the equation cu(no3)2 = cuo + no2 +. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for K2S + Cu(NO3)2 = KNO3 + CuS Iron Copper Ii Nitrate Balanced Equation See examples, instructions and tips. Consider the following sr reaction between iron metal and copper(ii) nitrate: Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The. Iron Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] iron filings added to copper(II) sulfate in solution (assume Iron Copper Ii Nitrate Balanced Equation In the activity series, fe is more reactive than cu, so the reaction. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Enter an equation of. Iron Copper Ii Nitrate Balanced Equation.
From www.meritnation.com
Write balanced chemical equations for the conversions A,B C A Copper Iron Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. See examples, instructions and tips. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. Consider the following sr reaction between iron metal and copper(ii) nitrate: In. Iron Copper Ii Nitrate Balanced Equation.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Iron Copper Ii Nitrate Balanced Equation Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Enter an equation of an ionic chemical equation and press the balance button. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. The balanced equation will be calculated along with the. Iron metal is added to a solution of copper (ii) nitrate express your answer. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED 1. When copper (II) chloride reacts with sodium nitrate, copper Iron Copper Ii Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. The balanced equation will be calculated along with the.. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
Equation for Fe(NO3)2 + H2O Iron (II) nitrate + Water YouTube Iron Copper Ii Nitrate Balanced Equation Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. The balanced equation will be calculated along with the. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Enter a chemical equation and press the balance. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Balance C5H9O + O2 = CO2 + H2O YouTube Iron Copper Ii Nitrate Balanced Equation In the activity series, fe is more reactive than cu, so the reaction. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The balanced equation will be calculated along with the. In this video we'll balance the equation cu(no3)2 =. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the Iron Copper Ii Nitrate Balanced Equation Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. See examples, instructions and tips. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED A balanced net ionic equation for chromium (III) nitrate and Iron Copper Ii Nitrate Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. First, we need to check if fe will displace cu in the. Iron Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Iron Copper Ii Nitrate Balanced Equation In the activity series, fe is more reactive than cu, so the reaction. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. See examples, instructions and tips. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Consider the following sr reaction between iron metal and. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write a word equation and a skeleton equation for the chemical Iron Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and. Enter an equation of an ionic chemical equation and press the balance button.. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Iron Copper Ii Nitrate Balanced Equation Consider the following sr reaction between iron metal and copper(ii) nitrate: The balanced equation will be calculated along with the. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. In the activity series, fe is more reactive than cu, so the reaction. Enter a chemical equation and press the balance button to get the balanced. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cu(NO3)2 Copper (II) nitrate Iron Copper Ii Nitrate Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Enter an equation of an ionic chemical equation and press the balance button. Identify the oxidation numbers, determine the changes in oxidation. Iron Copper Ii Nitrate Balanced Equation.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Iron Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Consider the following sr reaction between iron metal and copper(ii) nitrate: In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. The equation above indicates that one mole of solid copper is reacting with. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Write the Formula for Iron (II) nitrate YouTube Iron Copper Ii Nitrate Balanced Equation Consider the following sr reaction between iron metal and copper(ii) nitrate: Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. The balanced equation will be calculated along with the. Iron metal is added to a solution of copper (ii) nitrate express your answer as. Iron Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Iron Copper Ii Nitrate Balanced Equation Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. Enter a chemical equation and press the balance button to get the balanced equation and the. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
A solution of Iron (II) nitrate is poured into a sodium sulfide Iron Copper Ii Nitrate Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. Enter an equation of an ionic chemical equation and press the balance button. In the activity series, fe is more reactive than cu, so the reaction. The equation above indicates that. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Part 1 [Copper metal to Copper (II) nitrate] 2pts. Balanced Iron Copper Ii Nitrate Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. The balanced equation will be calculated along with the. First, we need to check if fe will. Iron Copper Ii Nitrate Balanced Equation.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Iron Copper Ii Nitrate Balanced Equation Iron metal is added to a solution of copper (ii) nitrate express your answer as a balanced chemical equation. First, we need to check if fe will displace cu in the copper(ii) nitrate solution. The balanced equation will be calculated along with the. Identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation. Iron Copper Ii Nitrate Balanced Equation.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Iron Copper Ii Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Fe(s) + cu(no 3) 2 (aq) → fe(no 3) 2. First, we need to. Iron Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced molecular equation, including phases, for Iron Copper Ii Nitrate Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. In this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. In the activity series, fe is more reactive than cu, so the reaction. The balanced equation will be calculated along with the. Enter an equation of an. Iron Copper Ii Nitrate Balanced Equation.