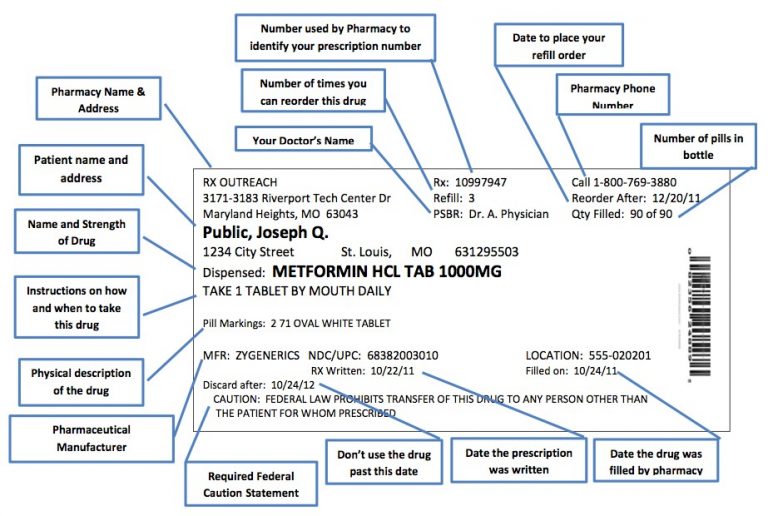
Dispensing Labels Legal Requirements . This paragraph applies only to prescription drug. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (2) the controlled substance listed in. (d) labeling requirements for new and more recently approved prescription drug products. Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to.
from aplmed.com
This paragraph applies only to prescription drug. (d) labeling requirements for new and more recently approved prescription drug products. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. (2) the controlled substance listed in. Ultimately, these acts established the criteria for labeling a medication as a.
4. Documenting Medications (MAR). Aplmed Academy
Dispensing Labels Legal Requirements Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. This paragraph applies only to prescription drug. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. (d) labeling requirements for new and more recently approved prescription drug products. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ultimately, these acts established the criteria for labeling a medication as a. (2) the controlled substance listed in. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c.
From ambitiousmares.blogspot.com
33 Pharmacy Dispensing Label Labels Design Ideas 2020 Dispensing Labels Legal Requirements Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Carton and container labeling are designed to promote safe dispensing, administration, and. Dispensing Labels Legal Requirements.
From www.slideshare.net
Basic principles of compounding and dispensingprescription, labeling… Dispensing Labels Legal Requirements (2) the controlled substance listed in. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. Ultimately, these acts established the criteria for labeling a medication as a. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This paragraph applies. Dispensing Labels Legal Requirements.
From nhslabels.co.uk
Dispensing Labels PM Strategic Sourcing Dispensing Labels Legal Requirements Ultimately, these acts established the criteria for labeling a medication as a. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This paragraph applies only to prescription drug. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Requirements governing the labeling and. Dispensing Labels Legal Requirements.
From dandelionsandthings.blogspot.com
33 The Dispensing Label On An Outpatient Pharmacy Prescription Requires Dispensing Labels Legal Requirements Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This paragraph applies only. Dispensing Labels Legal Requirements.
From armymedical.tpub.com
Figure 214. Sample label format for prepackaging dispensing units Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (d) labeling requirements for new and more recently approved prescription drug products. Ultimately, these acts established the criteria for labeling a medication as a.. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
30 Dispensing Label Requirements Uk Labels Design Ideas 2020 Dispensing Labels Legal Requirements (2) the controlled substance listed in. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. (d) labeling requirements for new and more recently approved prescription drug products. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Requirements governing the labeling and packaging of controlled substances. Dispensing Labels Legal Requirements.
From visionsupply.com.au
Buy Online Dispensing label AMFAC (500 Labels) at Best Price Dispensing Labels Legal Requirements Ultimately, these acts established the criteria for labeling a medication as a. (2) the controlled substance listed in. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. (d) labeling requirements for new and. Dispensing Labels Legal Requirements.
From www.slideshare.net
Basic principles of compounding and dispensingprescription, labeling… Dispensing Labels Legal Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ultimately, these acts established the criteria for labeling a medication as a. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. Carton and container labeling are designed to promote safe. Dispensing Labels Legal Requirements.
From www.studocu.com
Dispensing Essentials Legal requirements for prescriptions for POMs a Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. (d) labeling requirements for new and more recently approved prescription drug products. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This paragraph applies only to prescription drug. Ultimately, these acts established the. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
30 Dispensing Label Requirements Uk Labels Design Ideas 2020 Dispensing Labels Legal Requirements The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. (2) the controlled substance listed in. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ultimately, these acts established the criteria for labeling a medication as a. Requirements governing the labeling and packaging of. Dispensing Labels Legal Requirements.
From help.minfos.com.au
Dispense Totals Labels Help Centre Dispensing Labels Legal Requirements Ultimately, these acts established the criteria for labeling a medication as a. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. (2) the controlled substance listed in. This paragraph applies only to prescription drug. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for. Dispensing Labels Legal Requirements.
From www.slideserve.com
PPT DISPENSING & LABELING MEDICATION PowerPoint Presentation ID2699222 Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing and dispensing of controlled substances is. Dispensing Labels Legal Requirements.
From brennad-images.blogspot.com
Printable Prescription Warning Labels / Ers Solutions Pharmacy Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
30 Dispensing Label Requirements Uk Labels Design Ideas 2020 Dispensing Labels Legal Requirements The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Ultimately, these acts established the criteria for labeling a medication as a. This paragraph applies only to prescription drug. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. (d) labeling requirements for new and more recently. Dispensing Labels Legal Requirements.
From dokumen.tips
(PDF) Printer dispensing systems Marking with labels DOKUMEN.TIPS Dispensing Labels Legal Requirements This paragraph applies only to prescription drug. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ultimately, these acts established the criteria for labeling a medication as a. (d) labeling requirements for new and more recently approved prescription drug products. The responsibility for the proper prescribing and dispensing of controlled. Dispensing Labels Legal Requirements.
From www.scribd.com
Optimizing Dispensing Labels Guidelines for Clear Communication of Dispensing Labels Legal Requirements Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. (d) labeling requirements for new and more recently approved prescription drug products. Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,.. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
30 Dispensing Label Requirements Uk Labels Design Ideas 2020 Dispensing Labels Legal Requirements The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. (2) the controlled substance listed in. Human. Dispensing Labels Legal Requirements.
From www.slideserve.com
PPT DISPENSING & LABELING MEDICATION PowerPoint Presentation ID2699222 Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Ultimately, these acts established the criteria for labeling a medication as a. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Requirements governing the labeling and packaging of controlled substances pursuant to sections. Dispensing Labels Legal Requirements.
From www.mdpi.com
Pharmacy Free FullText Provision of Bilingual Dispensing Labels to Dispensing Labels Legal Requirements (2) the controlled substance listed in. Ultimately, these acts established the criteria for labeling a medication as a. (d) labeling requirements for new and more recently approved prescription drug products. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. The responsibility for the proper prescribing and dispensing of controlled substances is upon. Dispensing Labels Legal Requirements.
From www.slideserve.com
PPT DISPENSING & LABELING MEDICATION PowerPoint Presentation ID2699222 Dispensing Labels Legal Requirements Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. (d) labeling requirements for new and more recently approved prescription drug products. Human prescription drug labeling (1) contains a summary of the essential scientific. Dispensing Labels Legal Requirements.
From www.pharma-packaging.uk
Dispensing Labels Pharma Packaging Dispensing Labels Legal Requirements (d) labeling requirements for new and more recently approved prescription drug products. (2) the controlled substance listed in. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ultimately, these acts established the criteria for. Dispensing Labels Legal Requirements.
From www.totalpharmacy.com.au
Dispensing Labels — Total Pharmacy Dispensing Labels Legal Requirements (d) labeling requirements for new and more recently approved prescription drug products. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. This paragraph applies only to prescription drug. (2) the controlled substance listed in. Requirements governing. Dispensing Labels Legal Requirements.
From www.slideserve.com
PPT DISPENSING & LABELING MEDICATION PowerPoint Presentation ID2699222 Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. This paragraph applies only to prescription drug. (d) labeling requirements for new and more recently approved prescription drug products. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Requirements governing the labeling and. Dispensing Labels Legal Requirements.
From www.studocu.com
9. Dispensing (II) Legal Ethical Considerations LEGAL and ETHICAL Dispensing Labels Legal Requirements (d) labeling requirements for new and more recently approved prescription drug products. Requirements governing the labeling and packaging of controlled substances pursuant to sections 1305 and 1008(d) of the act (21 u.s.c. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. This paragraph applies only to prescription drug. (2) the controlled substance. Dispensing Labels Legal Requirements.
From purehealthsolutions.com.au
Personalised Practitioner Dispensing Labels Dispensing Labels Legal Requirements The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Ultimately, these acts established the criteria for labeling a medication as a. This paragraph applies only to prescription drug. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (2) the controlled substance listed in.. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
33 Pharmacy Dispensing Label Labels Design Ideas 2020 Dispensing Labels Legal Requirements (d) labeling requirements for new and more recently approved prescription drug products. This paragraph applies only to prescription drug. (2) the controlled substance listed in. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing. Dispensing Labels Legal Requirements.
From www.leadinglabel.co.nz
Dispensary Labels NZ Pharmaceutical Labels Christchurch Dispensing Labels Legal Requirements The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Ultimately, these acts established the criteria for labeling a medication as a. This paragraph applies only to prescription drug. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. (d) labeling requirements for new and more recently. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
30 Dispensing Label Requirements Uk Labels Design Ideas 2020 Dispensing Labels Legal Requirements (d) labeling requirements for new and more recently approved prescription drug products. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This paragraph applies only to prescription drug. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. (2) the controlled substance listed in.. Dispensing Labels Legal Requirements.
From eqmd.net
EQMD, Inc. Physician Medication Dispensing Software Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. This paragraph applies only to prescription drug. Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. (d) labeling requirements for new and more recently. Dispensing Labels Legal Requirements.
From www.slideshare.net
Pharmaceutical labelling PPT Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ultimately, these acts established the criteria for labeling. Dispensing Labels Legal Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Dispensing Labels Legal Requirements (d) labeling requirements for new and more recently approved prescription drug products. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. Ultimately, these acts established the criteria for labeling a medication as a. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Requirements governing the. Dispensing Labels Legal Requirements.
From www.stirlingfildes.com.au
StirlingFildes Printing, Packaging and Consumables Dispensing Labels Legal Requirements Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Ultimately, these acts established the criteria for labeling a medication as a. (2) the controlled substance listed in. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. (d) labeling requirements for new and more recently approved. Dispensing Labels Legal Requirements.
From aplmed.com
4. Documenting Medications (MAR). Aplmed Academy Dispensing Labels Legal Requirements Ultimately, these acts established the criteria for labeling a medication as a. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. (d) labeling requirements for new and more recently approved prescription drug products. Requirements. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
30 Dispensing Label Requirements Uk Labels Design Ideas 2020 Dispensing Labels Legal Requirements (d) labeling requirements for new and more recently approved prescription drug products. Ultimately, these acts established the criteria for labeling a medication as a. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (2) the controlled substance listed in. Requirements governing the labeling and packaging of controlled substances pursuant to. Dispensing Labels Legal Requirements.
From ambitiousmares.blogspot.com
30 Dispensing Label Requirements Uk Labels Design Ideas 2020 Dispensing Labels Legal Requirements Ultimately, these acts established the criteria for labeling a medication as a. The responsibility for the proper prescribing and dispensing of controlled substances is upon the prescribing practitioner,. This paragraph applies only to prescription drug. Carton and container labeling are designed to promote safe dispensing, administration, and use of the medicine to. Requirements governing the labeling and packaging of controlled. Dispensing Labels Legal Requirements.