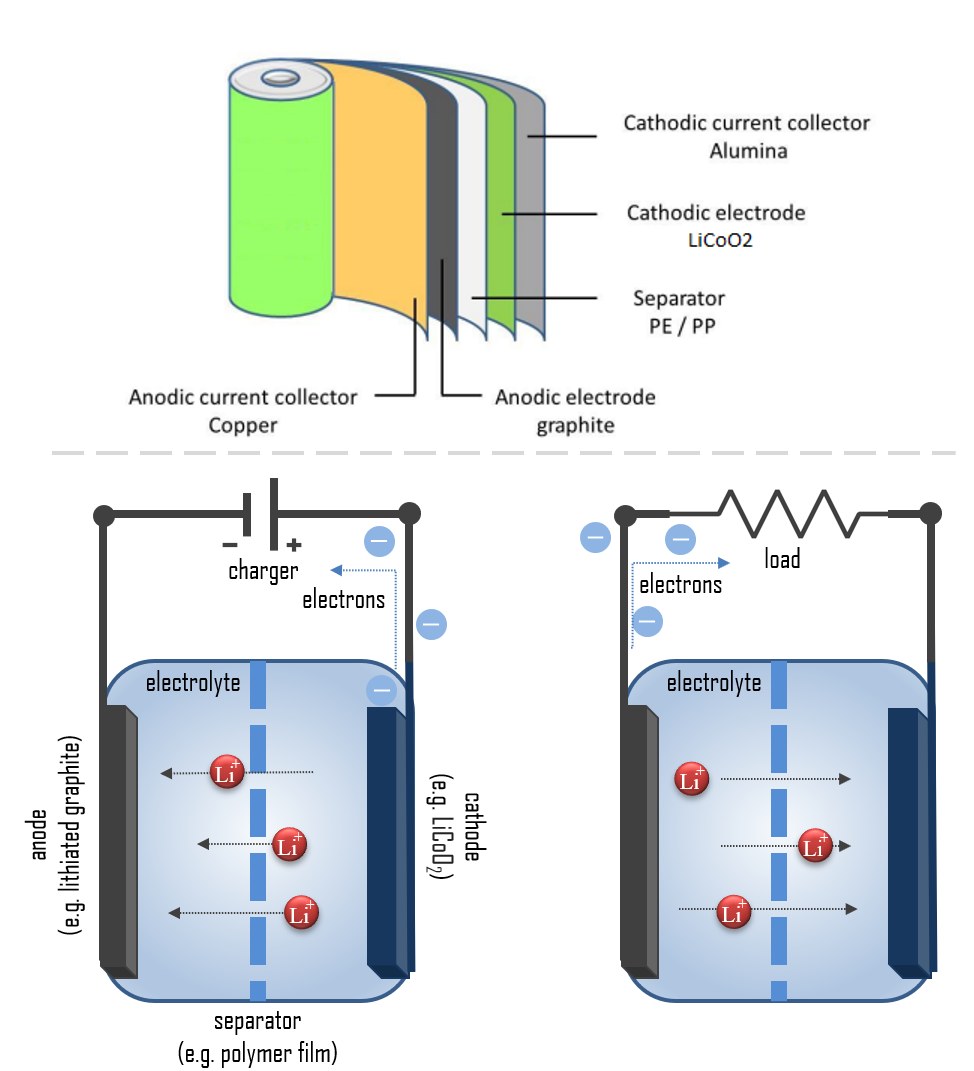
Battery Electrode Reaction . Reduction takes place at the cathode. Disposable, or primary, batteries, in which the electrode reactions are effectively. there are two basic kinds of batteries: one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Oxidation takes place at the anode. The materials in the electrodes are completely.
from www.electricity-magnetism.org
one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. The materials in the electrodes are completely. there are two basic kinds of batteries: with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Disposable, or primary, batteries, in which the electrode reactions are effectively. Reduction takes place at the cathode. Oxidation takes place at the anode.
Lithiumion Battery How it works Reaction, Anode & Cathode
Battery Electrode Reaction there are two basic kinds of batteries: with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Oxidation takes place at the anode. Reduction takes place at the cathode. there are two basic kinds of batteries: The materials in the electrodes are completely. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Disposable, or primary, batteries, in which the electrode reactions are effectively.
From classnotes.org.in
Batteries Chemistry, Class 12, Electro Chemistry Battery Electrode Reaction with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Reduction takes place at the cathode. there are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical. Battery Electrode Reaction.
From wisc.pb.unizin.org
Day 41 Electrolysis; Commercial Batteries Chemistry 109, Fall 2020 Battery Electrode Reaction The materials in the electrodes are completely. Reduction takes place at the cathode. there are two basic kinds of batteries: Oxidation takes place at the anode. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode, connects to the positive end of the battery and is where. Battery Electrode Reaction.
From www.researchgate.net
The principle of the lithiumion battery (LiB) showing the Battery Electrode Reaction with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Reduction takes place at the cathode. there are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical. Battery Electrode Reaction.
From www.researchgate.net
Schematic illustration of a composite electrode in lithiumion Battery Electrode Reaction Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Reduction takes place at the cathode. Oxidation takes place at the anode. The materials in the. Battery Electrode Reaction.
From www.researchgate.net
Schematic of the lithiumion battery with the graphite anode and LiCoO2 Battery Electrode Reaction with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. The materials in the electrodes are completely. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. there are two basic. Battery Electrode Reaction.
From arstechnica.com
New electrode material could lead to rechargeable sodium batteries Battery Electrode Reaction The materials in the electrodes are completely. Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. with the chemical intercalation reactions on metal disulfides. Battery Electrode Reaction.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry Battery Electrode Reaction one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. The materials in the electrodes are completely. Reduction takes place at the cathode. Disposable, or primary, batteries, in which the electrode reactions are effectively. with the. Battery Electrode Reaction.
From www.researchgate.net
Schematic representation of the discharge of a Li +ion battery a Battery Electrode Reaction Oxidation takes place at the anode. Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. there are two basic kinds of batteries: The materials. Battery Electrode Reaction.
From giofguzvb.blob.core.windows.net
Electrochemical Battery Reaction at Justin Carlson blog Battery Electrode Reaction Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Oxidation takes place at the anode. there are two basic kinds of batteries: Reduction takes. Battery Electrode Reaction.
From www.alamy.com
Early battery, working by chemical reaction, with zinc strip as Battery Electrode Reaction The materials in the electrodes are completely. there are two basic kinds of batteries: one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Reduction takes place at the cathode. with the chemical intercalation reactions. Battery Electrode Reaction.
From giofguzvb.blob.core.windows.net
Electrochemical Battery Reaction at Justin Carlson blog Battery Electrode Reaction Oxidation takes place at the anode. there are two basic kinds of batteries: The materials in the electrodes are completely. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons. Battery Electrode Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Battery Electrode Reaction there are two basic kinds of batteries: with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Oxidation takes place at the anode. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is. Battery Electrode Reaction.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Battery Electrode Reaction Reduction takes place at the cathode. there are two basic kinds of batteries: The materials in the electrodes are completely. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Oxidation takes place at the anode.. Battery Electrode Reaction.
From saylordotorg.github.io
Electrochemistry Battery Electrode Reaction Disposable, or primary, batteries, in which the electrode reactions are effectively. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Reduction takes place at the cathode. The materials in the electrodes are completely. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current. Battery Electrode Reaction.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in Battery Electrode Reaction The materials in the electrodes are completely. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Oxidation takes place at the. Battery Electrode Reaction.
From exyntiuij.blob.core.windows.net
Electrochemical Batteries Use at Jansen blog Battery Electrode Reaction Reduction takes place at the cathode. there are two basic kinds of batteries: Oxidation takes place at the anode. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter). Battery Electrode Reaction.
From www.biolinscientific.com
Wettability of electrodes Electrode calendering in Liion batteries Battery Electrode Reaction Disposable, or primary, batteries, in which the electrode reactions are effectively. Reduction takes place at the cathode. The materials in the electrodes are completely. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. there are. Battery Electrode Reaction.
From saylordotorg.github.io
Electrochemistry Battery Electrode Reaction Disposable, or primary, batteries, in which the electrode reactions are effectively. The materials in the electrodes are completely. Reduction takes place at the cathode. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. there are two basic kinds of batteries: one electrode, known as the cathode, connects to the positive end of. Battery Electrode Reaction.
From www.hidenanalytical.com
Cathode Studies New Opportunities in LiIon Batteries Battery Electrode Reaction there are two basic kinds of batteries: Reduction takes place at the cathode. Oxidation takes place at the anode. The materials in the electrodes are completely. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode, connects to the positive end of the battery and is where. Battery Electrode Reaction.
From semcouniversity.com
How the three electrode system works Semco University Semco Battery Electrode Reaction The materials in the electrodes are completely. Oxidation takes place at the anode. Reduction takes place at the cathode. there are two basic kinds of batteries: with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode,. Battery Electrode Reaction.
From news.mit.edu
How electrodes charge and discharge MIT News Massachusetts Battery Electrode Reaction one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. The materials in the electrodes are completely. there are two basic kinds of batteries: Reduction takes place at the cathode. Disposable, or primary, batteries, in which. Battery Electrode Reaction.
From www.electricity-magnetism.org
Lithiumion Battery How it works Reaction, Anode & Cathode Battery Electrode Reaction Oxidation takes place at the anode. Disposable, or primary, batteries, in which the electrode reactions are effectively. there are two basic kinds of batteries: one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Reduction takes. Battery Electrode Reaction.
From www.jeh-tech.com
Batteries notes Battery Electrode Reaction one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Oxidation takes place at the anode. there are two basic kinds of batteries: The materials in the electrodes are completely. Reduction takes place at the cathode.. Battery Electrode Reaction.
From wiredbemerson.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Battery Electrode Reaction Reduction takes place at the cathode. The materials in the electrodes are completely. Disposable, or primary, batteries, in which the electrode reactions are effectively. there are two basic kinds of batteries: Oxidation takes place at the anode. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode,. Battery Electrode Reaction.
From www.researchgate.net
Schematic illustration of a lithiumion battery during discharge/charge Battery Electrode Reaction The materials in the electrodes are completely. there are two basic kinds of batteries: Reduction takes place at the cathode. Disposable, or primary, batteries, in which the electrode reactions are effectively. Oxidation takes place at the anode. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves. Battery Electrode Reaction.
From saylordotorg.github.io
Electrochemistry Battery Electrode Reaction with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. there are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. Battery Electrode Reaction.
From www.slideserve.com
PPT The Lead Acid Electric Battery PowerPoint Presentation, free Battery Electrode Reaction one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. there are two basic kinds of batteries: with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Reduction takes place at. Battery Electrode Reaction.
From giocvjnlx.blob.core.windows.net
Surrounds Electrodes In A Battery To Help Produce Current at Renee Battery Electrode Reaction with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. The materials in the electrodes are completely. Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery. Battery Electrode Reaction.
From www.researchgate.net
Cell reaction for ZnMnO 2 alkaline battery Electrode Electrode & cell Battery Electrode Reaction one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Reduction takes place at the cathode. Oxidation takes place at the anode. The materials in the electrodes are completely. Disposable, or primary, batteries, in which the electrode. Battery Electrode Reaction.
From saylordotorg.github.io
Electrochemistry Battery Electrode Reaction The materials in the electrodes are completely. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Oxidation takes place at the anode. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when. Battery Electrode Reaction.
From www.youtube.com
Electrochemical Reaction in Lithium Ion Battery YouTube Battery Electrode Reaction Disposable, or primary, batteries, in which the electrode reactions are effectively. Reduction takes place at the cathode. The materials in the electrodes are completely. Oxidation takes place at the anode. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode, connects to the positive end of the battery. Battery Electrode Reaction.
From www.batterypowertips.com
What is a cathode? Battery Power Tips Battery Electrode Reaction Disposable, or primary, batteries, in which the electrode reactions are effectively. Reduction takes place at the cathode. there are two basic kinds of batteries: The materials in the electrodes are completely. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. one electrode, known as the cathode, connects to the positive end of. Battery Electrode Reaction.
From www.youtube.com
Electrochemistry / Secondary Cells /Nickel Cadmium battery/Leadacid Battery Electrode Reaction with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Reduction takes place at the cathode. there are two basic kinds of batteries: The materials in the electrodes are completely. Oxidation takes place at the anode. one electrode, known as the cathode, connects to the positive end of the battery and is where. Battery Electrode Reaction.
From www.electricity-magnetism.org
Nickelcadmium Battery How it works Reaction, Anode, Cathode Battery Electrode Reaction Reduction takes place at the cathode. with the chemical intercalation reactions on metal disulfides in place, whittingham 8 demonstrated the. Disposable, or primary, batteries, in which the electrode reactions are effectively. The materials in the electrodes are completely. Oxidation takes place at the anode. there are two basic kinds of batteries: one electrode, known as the cathode,. Battery Electrode Reaction.
From www.e-batterystore.com
Polarization and electromotive force of leadacid battery electrodes Battery Electrode Reaction one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which is when the. Disposable, or primary, batteries, in which the electrode reactions are effectively. The materials in the electrodes are completely. there are two basic kinds of batteries: . Battery Electrode Reaction.