Suitable Indicator In The Titration Of Naoh And Ch3Cooh . the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. This page assumes that you know. In the strong acid titration, use of any of the three. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Click here to review your answer to this exercise. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3.
from www.numerade.com
the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Click here to review your answer to this exercise. In the strong acid titration, use of any of the three. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid.
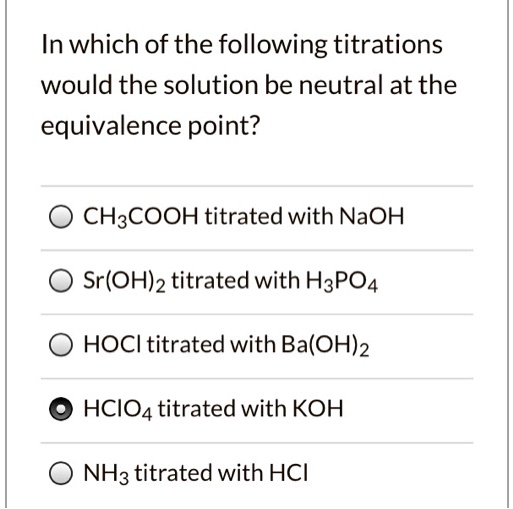
SOLVED In which of the following titrations would the solution be
Suitable Indicator In The Titration Of Naoh And Ch3Cooh suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. Click here to review your answer to this exercise. the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. In the strong acid titration, use of any of the three. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh.
From exyvzagmg.blob.core.windows.net
Best Indicator For Titration Of Acetic Acid With Naoh at Matthew Taylor Suitable Indicator In The Titration Of Naoh And Ch3Cooh suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. In the strong acid titration, use of any of the three. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. the titration curves shown in illustrate the choice. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From slideplayer.com
Experiment No. 3 Preparation and standardization of approximately 0.1N Suitable Indicator In The Titration Of Naoh And Ch3Cooh suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. the titration curves shown in illustrate the choice of a suitable indicator for specific. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
SOLVED In which of the following titrations would the solution be Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. This page assumes that you know. table 15.7.1 shows a detailed sequence of changes in the ph of. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.vrogue.co
Standardization Of Naoh Acid Base Titration Objective vrogue.co Suitable Indicator In The Titration Of Naoh And Ch3Cooh In the strong acid titration, use of any of the three. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. the titration curves shown in figure 14.20 illustrate the choice of a. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.youtube.com
PCAT Titration Curve of Weak Acid Strong Base (CH3COOH and NaOH Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. This page assumes that you know. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.youtube.com
Suitable indicator for titration of strong acid with strong base YouTube Suitable Indicator In The Titration Of Naoh And Ch3Cooh Click here to review your answer to this exercise. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. In the strong acid titration, use of any of the three. This page assumes that you know. the titration curves shown in figure 14.20 illustrate. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From socratic.org
Mixing up equal volumes of 0.1 M NaOH and 0.1 M CH3COOH yields a Suitable Indicator In The Titration Of Naoh And Ch3Cooh table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. Click here to review your answer to this exercise. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. the titration curves shown in. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
VIDEO solution A series of titrations of lactic acid, CH3CH(OH) COOH Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. Click here to review your answer to this exercise. You constructed a titration curve for this titration in practice exercise 9.2 and. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
SOLVED The following data is from a conductometric titration of 10.00 Suitable Indicator In The Titration Of Naoh And Ch3Cooh Click here to review your answer to this exercise. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. In the strong acid titration, use of any. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Suitable Indicator In The Titration Of Naoh And Ch3Cooh table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Click here to review your answer to this exercise. to minimize a determinate titration error, the. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From pubs.sciepub.com
Figure 8A. Plot of the titration of weak acid (CH3COOH= 0.1M) with Suitable Indicator In The Titration Of Naoh And Ch3Cooh suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. This page assumes that you know. table 15.7.1 shows a detailed sequence of changes in the ph of a. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From slideplayer.com
Titration What is the concentration in moles/liter of a vinegar Suitable Indicator In The Titration Of Naoh And Ch3Cooh suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
SOLVED A 0.100 M solution of ethanoic acid, CH3COOH, has a pH of 2.87 Suitable Indicator In The Titration Of Naoh And Ch3Cooh suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. to minimize a determinate titration error, the. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From giolipqwa.blob.core.windows.net
Titration Curve Of Nh3 And Hcl at Huntington blog Suitable Indicator In The Titration Of Naoh And Ch3Cooh You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. In the strong acid titration, use of any of the three. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. the titration curves shown in figure 14.20 illustrate the choice of a. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.youtube.com
Conductometric Titration "Weak acid vs Strong base" Practical Suitable Indicator In The Titration Of Naoh And Ch3Cooh to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.toppr.com
Match the titrations given with the indicators used in them Titration Suitable Indicator In The Titration Of Naoh And Ch3Cooh In the strong acid titration, use of any of the three. Click here to review your answer to this exercise. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From pubs.sciepub.com
Figure 9. First derivative plot of the titration of weak acid (CH3COOH Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. Click here to review your answer to this exercise. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. . Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From slideplayer.com
Experiment No. 3 Preparation and standardization of approximately 0.1N Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From hxevdtmcs.blob.core.windows.net
Titration Curve Concentration Of Acid at Richard Chaires blog Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration, use of any of the three. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. Click here to review your answer to this exercise. the. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.youtube.com
How to Balance NaOH + CH3COOH = CH3COONa + H2O YouTube Suitable Indicator In The Titration Of Naoh And Ch3Cooh Click here to review your answer to this exercise. In the strong acid titration, use of any of the three. This page assumes that you know. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. the titration curves shown in illustrate the choice of a suitable indicator for. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.chegg.com
Solved TABLE 5.1 TITRATION OF CH3COOH NaOH concentration Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. This page assumes that you know. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh.. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.researchgate.net
Titration of 0.3 g etching solution (40 HNO3, 7 HF, and 30 CH3COOH Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. This. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.youtube.com
Which indicator is suitable for the titrations `{("Titration Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Suitable Indicator In The Titration Of Naoh And Ch3Cooh This page assumes that you know. In the strong acid titration, use of any of the three. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. the. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
SOLVED The reaction of NaOH with vinegar is shown in the equation Suitable Indicator In The Titration Of Naoh And Ch3Cooh the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. In the strong acid titration, use. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From askfilo.com
What is the suitable indicator for titration of NaOH and oxalic acid.. Suitable Indicator In The Titration Of Naoh And Ch3Cooh the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Click here to review your answer to this exercise. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. the titration curves shown in. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
SOLVED The following is a titration curve of a 20 mL solution of 0.10 Suitable Indicator In The Titration Of Naoh And Ch3Cooh to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. Click here to review your answer to this exercise. table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a titration with naoh. the graph shows the results. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From chart-studio.plotly.com
Acetic Acid (CH3COOH) + Sodium Hydroxide (NaOH) Titration scatter Suitable Indicator In The Titration Of Naoh And Ch3Cooh This page assumes that you know. In the strong acid titration, use of any of the three. Click here to review your answer to this exercise. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. You constructed a titration curve for this titration in. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Suitable Indicator In The Titration Of Naoh And Ch3Cooh This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph.. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.numerade.com
SOLVED A 0.100 M solution of ethanoic acid, CH3COOH, has a pH of 2.87 Suitable Indicator In The Titration Of Naoh And Ch3Cooh This page assumes that you know. Click here to review your answer to this exercise. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh.. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Suitable Indicator In The Titration Of Naoh And Ch3Cooh Click here to review your answer to this exercise. This page assumes that you know. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. In the strong acid. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.youtube.com
Acetic acid (ethanoic acid) and Sodium hydroxide reaction CH3COOH Suitable Indicator In The Titration Of Naoh And Ch3Cooh In the strong acid titration, use of any of the three. Click here to review your answer to this exercise. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From saylordotorg.github.io
AcidBase Titrations Suitable Indicator In The Titration Of Naoh And Ch3Cooh You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. table 15.7.1 shows a detailed sequence of changes in. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + CH3COOH = CH3COONa + H2O Suitable Indicator In The Titration Of Naoh And Ch3Cooh Click here to review your answer to this exercise. the titration curves shown in illustrate the choice of a suitable indicator for specific titrations. You constructed a titration curve for this titration in practice exercise 9.2 and practice exercise 9.3. In the strong acid titration, use of any of the three. the graph shows the results obtained using. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Suitable Indicator In The Titration Of Naoh And Ch3Cooh to minimize a determinate titration error, the indicator’s entire ph range must fall within the rapid change in ph. suggest a suitable indicator for the titration of 25.0 ml of 0.125 m nh 3 with 0.0625 m naoh. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m. Suitable Indicator In The Titration Of Naoh And Ch3Cooh.