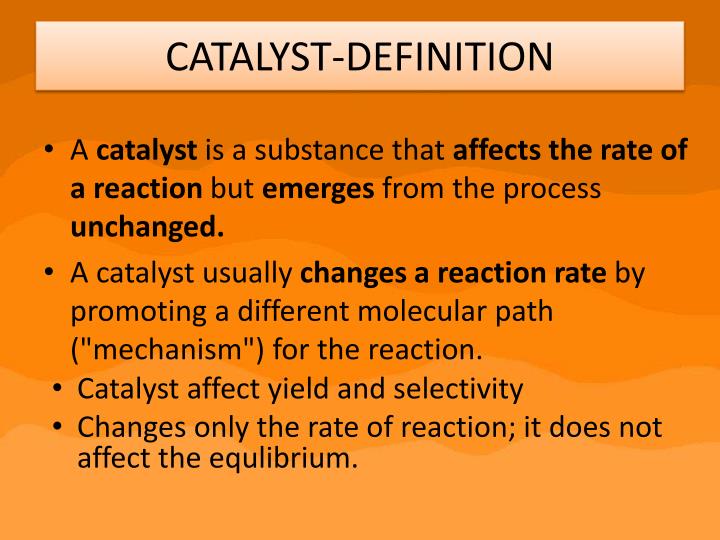
Does A Catalyst Affect K . A catalyst will provide a route for the reaction with a lower activation energy. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. What are catalysts, and how do they work in terms altering the parameters of a reaction? The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. A catalyst speeds up both the forward and back reactions by exactly the same amount. It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. Dynamic equilibrium is established when the rates of the. The effect of a catalyst. When the reaction has finished, you would have exactly the same mass of. How does a catalyst work?.
from www.slideserve.com
How does a catalyst work?. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. A catalyst speeds up both the forward and back reactions by exactly the same amount. Dynamic equilibrium is established when the rates of the. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. What are catalysts, and how do they work in terms altering the parameters of a reaction?
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint
Does A Catalyst Affect K What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. A catalyst speeds up both the forward and back reactions by exactly the same amount. Dynamic equilibrium is established when the rates of the. The effect of a catalyst. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst will provide a route for the reaction with a lower activation energy. How does a catalyst work?. It assumes that you are already familiar with basic ideas about the collision theory of reaction.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Does A Catalyst Affect K A catalyst will provide a route for the reaction with a lower activation energy. The effect of a catalyst. How does a catalyst work?. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst speeds up. Does A Catalyst Affect K.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Does A Catalyst Affect K What are catalysts, and how do they work in terms altering the parameters of a reaction? The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. It assumes that you are. Does A Catalyst Affect K.
From byjus.com
How does catalyst affect activation energy? Does A Catalyst Affect K A catalyst speeds up both the forward and back reactions by exactly the same amount. It assumes that you are already familiar with basic ideas about the collision theory of reaction. When the reaction has finished, you would have exactly the same mass of. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. The. Does A Catalyst Affect K.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Does A Catalyst Affect K This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst speeds up both the forward and back reactions by exactly the same amount. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes that you are already familiar with basic ideas about the. Does A Catalyst Affect K.
From slideplayer.com
*Le Châtelier’s Principle and Equilibrium ppt download Does A Catalyst Affect K It assumes that you are already familiar with basic ideas about the collision theory of reaction. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. A catalyst will provide a route for the reaction. Does A Catalyst Affect K.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Does A Catalyst Affect K A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. How does a catalyst work?. Suppose in the presence of a catalyst that the activation energy falls to 25 kj.. Does A Catalyst Affect K.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Does A Catalyst Affect K What are catalysts, and how do they work in terms altering the parameters of a reaction? Suppose in the presence of a catalyst that the activation energy falls to 25 kj. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. How does a catalyst work?. Adding a catalyst makes. Does A Catalyst Affect K.
From www.youtube.com
Identifying catalysts in a reaction YouTube Does A Catalyst Affect K It assumes that you are already familiar with basic ideas about the collision theory of reaction. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. Adding a catalyst makes absolutely no difference to the position. Does A Catalyst Affect K.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Does A Catalyst Affect K A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. What are catalysts, and how do they work in terms altering the parameters of a reaction? Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. It assumes that you. Does A Catalyst Affect K.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Does A Catalyst Affect K This page describes and explains the way that adding a catalyst affects the rate of a reaction. When the reaction has finished, you would have exactly the same mass of. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a substance which speeds up a reaction, but is chemically unchanged. Does A Catalyst Affect K.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Does A Catalyst Affect K The effect of a catalyst. A catalyst speeds up both the forward and back reactions by exactly the same amount. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst will provide a route for the. Does A Catalyst Affect K.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine Does A Catalyst Affect K A catalyst speeds up both the forward and back reactions by exactly the same amount. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Dynamic equilibrium is established when the rates of the. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst speeds. Does A Catalyst Affect K.
From hxeaspeqb.blob.core.windows.net
Why Do Catalysts Not Affect The Position Of Equilibrium at Erica Murphy Does A Catalyst Affect K It assumes that you are already familiar with basic ideas about the collision theory of reaction. Dynamic equilibrium is established when the rates of the. A catalyst will provide a route for the reaction with a lower activation energy. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. What. Does A Catalyst Affect K.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Does A Catalyst Affect K It assumes that you are already familiar with basic ideas about the collision theory of reaction. The effect of a catalyst. How does a catalyst work?. Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. When the reaction has finished, you would have exactly the same mass of. The. Does A Catalyst Affect K.
From www.thoughtco.com
Catalysis Definition in Chemistry Does A Catalyst Affect K This page describes and explains the way that adding a catalyst affects the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst speeds up both the forward and back reactions by exactly the same amount. It assumes that you are already familiar with basic ideas about the. Does A Catalyst Affect K.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine Does A Catalyst Affect K A catalyst speeds up both the forward and back reactions by exactly the same amount. A catalyst will provide a route for the reaction with a lower activation energy. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way that adding a catalyst affects the rate of. Does A Catalyst Affect K.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Does A Catalyst Affect K A catalyst speeds up both the forward and back reactions by exactly the same amount. The effect of a catalyst. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. Dynamic equilibrium is established when the rates of the. Suppose in the presence of a catalyst that the activation energy falls. Does A Catalyst Affect K.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Does A Catalyst Affect K The effect of a catalyst. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Dynamic equilibrium is established when the rates of the. Adding a catalyst makes absolutely no difference to the position of. Does A Catalyst Affect K.
From www.youtube.com
How does Catalyst affect the Rate of Chemical Reaction Lowering or Does A Catalyst Affect K When the reaction has finished, you would have exactly the same mass of. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst speeds up both the forward and back reactions by exactly the same amount.. Does A Catalyst Affect K.
From helecu.com
Factors Affecting Reaction Rates Chemistry Atoms First (2022) Does A Catalyst Affect K A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. Suppose in the presence of a catalyst that the activation energy falls to 25 kj. A catalyst speeds up both. Does A Catalyst Affect K.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Does A Catalyst Affect K A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. A catalyst will provide a route for the reaction with a lower activation energy. Dynamic equilibrium is established when the rates of the. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end. Does A Catalyst Affect K.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Does A Catalyst Affect K When the reaction has finished, you would have exactly the same mass of. Adding a catalyst makes absolutely no difference to the position of equilibrium, and le ch â telier 's principle does. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Dynamic equilibrium is established when the rates. Does A Catalyst Affect K.
From byjus.com
3. How does catalysts affect The equilibrium? Does A Catalyst Affect K A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. It assumes that you are already familiar with basic ideas about the collision theory of reaction. The effect of a catalyst. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction.. Does A Catalyst Affect K.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Does A Catalyst Affect K How does a catalyst work?. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. A catalyst will provide a route for the reaction with a lower activation energy. A catalyst speeds up both the forward and back reactions by exactly the same amount. Dynamic equilibrium is established when the. Does A Catalyst Affect K.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst Does A Catalyst Affect K How does a catalyst work?. This page describes and explains the way that adding a catalyst affects the rate of a reaction. When the reaction has finished, you would have exactly the same mass of. A catalyst speeds up both the forward and back reactions by exactly the same amount. Suppose in the presence of a catalyst that the activation. Does A Catalyst Affect K.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Does A Catalyst Affect K What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction. When the reaction has finished, you would have exactly the same mass of. This page describes and explains the way that adding a catalyst affects the rate of. Does A Catalyst Affect K.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine Does A Catalyst Affect K A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. A catalyst speeds up both the forward and back reactions by exactly the same amount. How does a catalyst work?. When the reaction has finished, you would have exactly the same mass of. Suppose in the presence of a catalyst. Does A Catalyst Affect K.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Does A Catalyst Affect K A catalyst speeds up both the forward and back reactions by exactly the same amount. What are catalysts, and how do they work in terms altering the parameters of a reaction? When the reaction has finished, you would have exactly the same mass of. How does a catalyst work?. The effect of a catalyst. Adding a catalyst makes absolutely no. Does A Catalyst Affect K.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Does A Catalyst Affect K It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst speeds up both the forward and back reactions by exactly the same amount. When the reaction has finished, you would have exactly the same mass of. Dynamic equilibrium is established when the rates of the. A catalyst is a substance which speeds. Does A Catalyst Affect K.
From www.semanticscholar.org
[PDF] Concepts of modern catalysis and Semantic Scholar Does A Catalyst Affect K It assumes that you are already familiar with basic ideas about the collision theory of reaction. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. A catalyst speeds up both the forward and back reactions by exactly the same amount. How does a catalyst work?. Suppose in the presence of. Does A Catalyst Affect K.
From www.savemyexams.com
Types of Catalyst CIE A Level Chemistry Revision Notes 2025 Does A Catalyst Affect K A catalyst speeds up both the forward and back reactions by exactly the same amount. The effect of a catalyst is seen in its ability to reduce the activation energy required for a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Adding a catalyst makes absolutely no difference to the. Does A Catalyst Affect K.
From www.nagwa.com
Question Video Determining How a Catalyst Affects the Energy Profile Does A Catalyst Affect K A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. What are catalysts, and how do they work in terms altering the parameters of a reaction? Suppose in the presence of a catalyst that the activation energy falls to 25 kj. This page describes and explains the way that adding. Does A Catalyst Affect K.
From byjus.com
Why do catalyst not affect equilibrium? Does A Catalyst Affect K A catalyst will provide a route for the reaction with a lower activation energy. What are catalysts, and how do they work in terms altering the parameters of a reaction? Suppose in the presence of a catalyst that the activation energy falls to 25 kj. A catalyst speeds up both the forward and the reverse reactions, so there is no. Does A Catalyst Affect K.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Does A Catalyst Affect K A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. This page describes and explains the way that adding a catalyst affects the rate of a reaction. How does a catalyst work?. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of. Does A Catalyst Affect K.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Does A Catalyst Affect K Dynamic equilibrium is established when the rates of the. It assumes that you are already familiar with basic ideas about the collision theory of reaction. How does a catalyst work?. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. Suppose in the presence of a catalyst that the activation. Does A Catalyst Affect K.