Does An Atom Have A Negative Charge . An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms contain negatively charged electrons and positively charged protons; By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. This means that the negative charge on an electron perfectly balances the positive charge on the proton. This effect of “resultant charge” can. In other words, a neutral atom must. The number of each determines the atom’s net charge. Positively charged atoms called cations are. However, the gain or loss of an electron. Electron, lightest stable subatomic particle known.
from www.slideserve.com
The number of each determines the atom’s net charge. In other words, a neutral atom must. Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. Positively charged atoms called cations are. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. However, the gain or loss of an electron. This means that the negative charge on an electron perfectly balances the positive charge on the proton. Atoms contain negatively charged electrons and positively charged protons; An atom that gains one or more electrons will exhibit a negative charge and is called an anion.
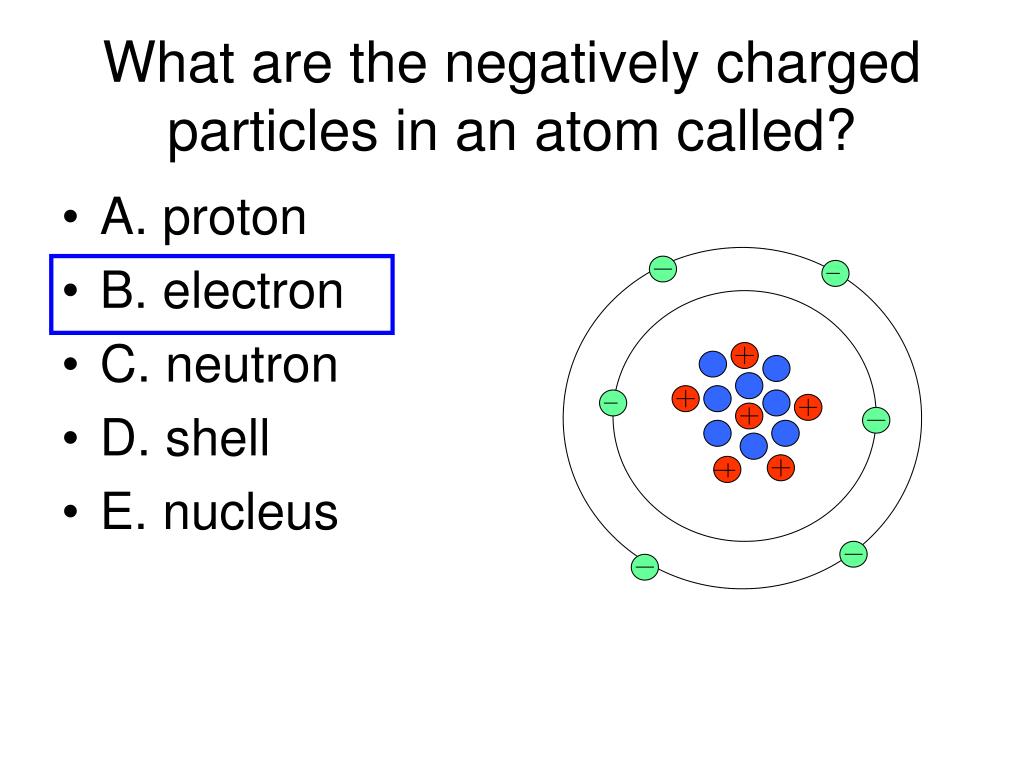
PPT What part of an atom is the arrow pointing to? PowerPoint
Does An Atom Have A Negative Charge By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. This means that the negative charge on an electron perfectly balances the positive charge on the proton. This effect of “resultant charge” can. Electron, lightest stable subatomic particle known. In other words, a neutral atom must. Positively charged atoms called cations are. Atoms contain negatively charged electrons and positively charged protons; However, the gain or loss of an electron. The number of each determines the atom’s net charge. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge.
From classnotes.ng
Electric Charges ClassNotes.ng Does An Atom Have A Negative Charge The number of each determines the atom’s net charge. This effect of “resultant charge” can. Positively charged atoms called cations are. Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. Atoms contain negatively charged electrons and positively charged protons; However, the gain or loss of an electron. Electron, lightest. Does An Atom Have A Negative Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Does An Atom Have A Negative Charge This effect of “resultant charge” can. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are. In other words, a neutral atom must. Electron, lightest stable subatomic particle known. Atoms contain negatively charged electrons and positively charged protons; This means that the negative charge on an. Does An Atom Have A Negative Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Does An Atom Have A Negative Charge Electron, lightest stable subatomic particle known. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. In other words, a neutral atom must. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. This effect of “resultant charge” can. This means. Does An Atom Have A Negative Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Does An Atom Have A Negative Charge Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. Positively charged atoms called cations are. The number of each determines the atom’s net charge. Electron, lightest stable subatomic particle known. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have. Does An Atom Have A Negative Charge.
From slideplayer.com
12.1 Atomic Structure. ppt download Does An Atom Have A Negative Charge An atom that gains one or more electrons will exhibit a negative charge and is called an anion. This effect of “resultant charge” can. In other words, a neutral atom must. This means that the negative charge on an electron perfectly balances the positive charge on the proton. However, the gain or loss of an electron. The number of each. Does An Atom Have A Negative Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Does An Atom Have A Negative Charge In other words, a neutral atom must. Atoms contain negatively charged electrons and positively charged protons; Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. This means that the negative charge on an electron perfectly balances the positive charge on the proton. This effect of “resultant charge” can. By. Does An Atom Have A Negative Charge.
From www.pinterest.com
Spectroscopy Electron configuration, Chemistry education, Protons Does An Atom Have A Negative Charge In other words, a neutral atom must. Positively charged atoms called cations are. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The number of each determines the atom’s net charge. An atom that gains one or more electrons will exhibit a negative charge and is called an. Does An Atom Have A Negative Charge.
From www.askiitians.com
Basic Properties of Electric Charge Study Material for IIT JEE Does An Atom Have A Negative Charge Atoms contain negatively charged electrons and positively charged protons; Electron, lightest stable subatomic particle known. Positively charged atoms called cations are. In other words, a neutral atom must. However, the gain or loss of an electron. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Some atoms have nearly eight electrons. Does An Atom Have A Negative Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Does An Atom Have A Negative Charge Electron, lightest stable subatomic particle known. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. However, the gain or loss of an electron. The number of each determines the atom’s. Does An Atom Have A Negative Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Does An Atom Have A Negative Charge In other words, a neutral atom must. Positively charged atoms called cations are. Electron, lightest stable subatomic particle known. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. However, the gain or loss of an electron. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled. Does An Atom Have A Negative Charge.
From www.nagwa.com
Question Video Identifying the Name of Negatively Charged Particles Does An Atom Have A Negative Charge Atoms contain negatively charged electrons and positively charged protons; By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. In other words, a neutral atom must. However, the gain or loss of an electron. An atom that gains one or more electrons will exhibit a negative charge. Does An Atom Have A Negative Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Does An Atom Have A Negative Charge This effect of “resultant charge” can. Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. However, the gain or loss of an electron. Some atoms have nearly eight electrons in their. Does An Atom Have A Negative Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Does An Atom Have A Negative Charge This means that the negative charge on an electron perfectly balances the positive charge on the proton. Electron, lightest stable subatomic particle known. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have. Does An Atom Have A Negative Charge.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry Does An Atom Have A Negative Charge Electron, lightest stable subatomic particle known. Positively charged atoms called cations are. This effect of “resultant charge” can. The number of each determines the atom’s net charge. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. An atom that gains one or more electrons will exhibit. Does An Atom Have A Negative Charge.
From www.thesciencehive.co.uk
Atomic Structure* — the science sauce Does An Atom Have A Negative Charge However, the gain or loss of an electron. Atoms contain negatively charged electrons and positively charged protons; Electron, lightest stable subatomic particle known. In other words, a neutral atom must. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. An atom that gains one or more electrons will. Does An Atom Have A Negative Charge.
From www.slideserve.com
PPT Atoms and Molecules PowerPoint Presentation, free download ID Does An Atom Have A Negative Charge Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. The number of each determines the atom’s net charge. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. An atom that gains one or more electrons. Does An Atom Have A Negative Charge.
From nasri-mm.blogspot.com
Nasri XII Multimedia STATIC ELECTRICITY Does An Atom Have A Negative Charge Electron, lightest stable subatomic particle known. This means that the negative charge on an electron perfectly balances the positive charge on the proton. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. Atoms contain negatively charged electrons and positively charged protons; Positively charged atoms called cations. Does An Atom Have A Negative Charge.
From www.mindomo.com
Energy Mind Map Does An Atom Have A Negative Charge In other words, a neutral atom must. The number of each determines the atom’s net charge. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. This means that the negative charge on an electron perfectly balances the positive charge on the proton. Some atoms have nearly eight electrons in their valence. Does An Atom Have A Negative Charge.
From slidetodoc.com
What is the relationship between atoms and elements Does An Atom Have A Negative Charge Electron, lightest stable subatomic particle known. However, the gain or loss of an electron. Positively charged atoms called cations are. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until. Does An Atom Have A Negative Charge.
From www.tpsearchtool.com
3 Estructura Atomica Proton Elementos Quimicos Images Does An Atom Have A Negative Charge Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. Electron, lightest stable subatomic particle known. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Positively charged atoms called cations are. An atom that gains one or more. Does An Atom Have A Negative Charge.
From www.sliderbase.com
Atoms, molecules and ions Presentation Chemistry Does An Atom Have A Negative Charge Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. This effect of “resultant charge” can. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. However, the gain or loss of an electron. Electron, lightest stable subatomic particle. Does An Atom Have A Negative Charge.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Does An Atom Have A Negative Charge Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. The number of each determines the atom’s net charge. In other words, a neutral atom must. Positively charged atoms called cations are. However, the gain or loss of an electron. Atoms contain negatively charged electrons and positively charged protons; By. Does An Atom Have A Negative Charge.
From www.expii.com
Atoms — Definition & Overview Expii Does An Atom Have A Negative Charge This effect of “resultant charge” can. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. In other words, a neutral atom must. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. However, the gain. Does An Atom Have A Negative Charge.
From infraredforhealth.com
How Do You Explain Negative Charge? Infrared for Health Does An Atom Have A Negative Charge However, the gain or loss of an electron. In other words, a neutral atom must. Positively charged atoms called cations are. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet.. Does An Atom Have A Negative Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Does An Atom Have A Negative Charge Atoms contain negatively charged electrons and positively charged protons; The number of each determines the atom’s net charge. This effect of “resultant charge” can. Electron, lightest stable subatomic particle known. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Ions that gain electrons, therefore have a negative resultant. Does An Atom Have A Negative Charge.
From www.slideshare.net
Atomic particles Does An Atom Have A Negative Charge Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. This means that the negative charge on an electron perfectly balances the positive charge on the proton. This effect. Does An Atom Have A Negative Charge.
From slideplayer.com
Ions and Ionic Compounds ppt download Does An Atom Have A Negative Charge Positively charged atoms called cations are. Atoms contain negatively charged electrons and positively charged protons; However, the gain or loss of an electron. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. In other words, a neutral atom must. Electron, lightest stable subatomic particle known. Ions that gain electrons, therefore have. Does An Atom Have A Negative Charge.
From maha22.weebly.com
Diagram of positive,negative atoms SCIENCE Does An Atom Have A Negative Charge Atoms contain negatively charged electrons and positively charged protons; Electron, lightest stable subatomic particle known. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. In other words, a neutral atom must. Positively charged atoms called cations are. Ions that gain electrons, therefore have a negative resultant charge, while ions that lose. Does An Atom Have A Negative Charge.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Does An Atom Have A Negative Charge Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. In other words, a neutral atom must. Atoms contain negatively charged electrons and positively charged protons; The number of each determines the atom’s net charge. This means that the negative charge on an electron perfectly balances the positive charge on. Does An Atom Have A Negative Charge.
From slideplayer.com
Atoms Vocab. ppt download Does An Atom Have A Negative Charge Electron, lightest stable subatomic particle known. However, the gain or loss of an electron. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. This effect of “resultant charge” can. Atoms contain negatively charged electrons and positively charged protons; The number of each determines the atom’s net charge. In. Does An Atom Have A Negative Charge.
From www.slideserve.com
PPT Types of Chemical Bonds PowerPoint Presentation, free download Does An Atom Have A Negative Charge Ions that gain electrons, therefore have a negative resultant charge, while ions that lose electrons have a positive resultant charge. The number of each determines the atom’s net charge. However, the gain or loss of an electron. This means that the negative charge on an electron perfectly balances the positive charge on the proton. By definition, atoms are neutral entities. Does An Atom Have A Negative Charge.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation ID4438872 Does An Atom Have A Negative Charge By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. In other words, a neutral atom must. However, the gain or loss of an electron. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. This effect of “resultant. Does An Atom Have A Negative Charge.
From www.slideserve.com
PPT What part of an atom is the arrow pointing to? PowerPoint Does An Atom Have A Negative Charge Electron, lightest stable subatomic particle known. This effect of “resultant charge” can. This means that the negative charge on an electron perfectly balances the positive charge on the proton. However, the gain or loss of an electron. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. By definition,. Does An Atom Have A Negative Charge.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Does An Atom Have A Negative Charge Atoms contain negatively charged electrons and positively charged protons; In other words, a neutral atom must. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. The number of. Does An Atom Have A Negative Charge.
From www.the-sun.com
Which subatomic particle has a negative charge? The US Sun Does An Atom Have A Negative Charge However, the gain or loss of an electron. In other words, a neutral atom must. By definition, atoms are neutral entities because the positive charge of the nucleus is cancelled by the negative charge of the electron cloud. Positively charged atoms called cations are. Electron, lightest stable subatomic particle known. Ions that gain electrons, therefore have a negative resultant charge,. Does An Atom Have A Negative Charge.