Catalyst Examples . A catalyst is a material that speeds up chemical reactions. When heated by itself, a sugar cube (sucrose) melts at 185°c. Some common examples of catalysis. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. Also, discover how catalysts can be used figuratively to describe social or personal changes. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. How to burn a sugar cube. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work.
from www.mdpi.com
When heated by itself, a sugar cube (sucrose) melts at 185°c. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. A catalyst is a material that speeds up chemical reactions. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. Also, discover how catalysts can be used figuratively to describe social or personal changes. Some common examples of catalysis. How to burn a sugar cube.
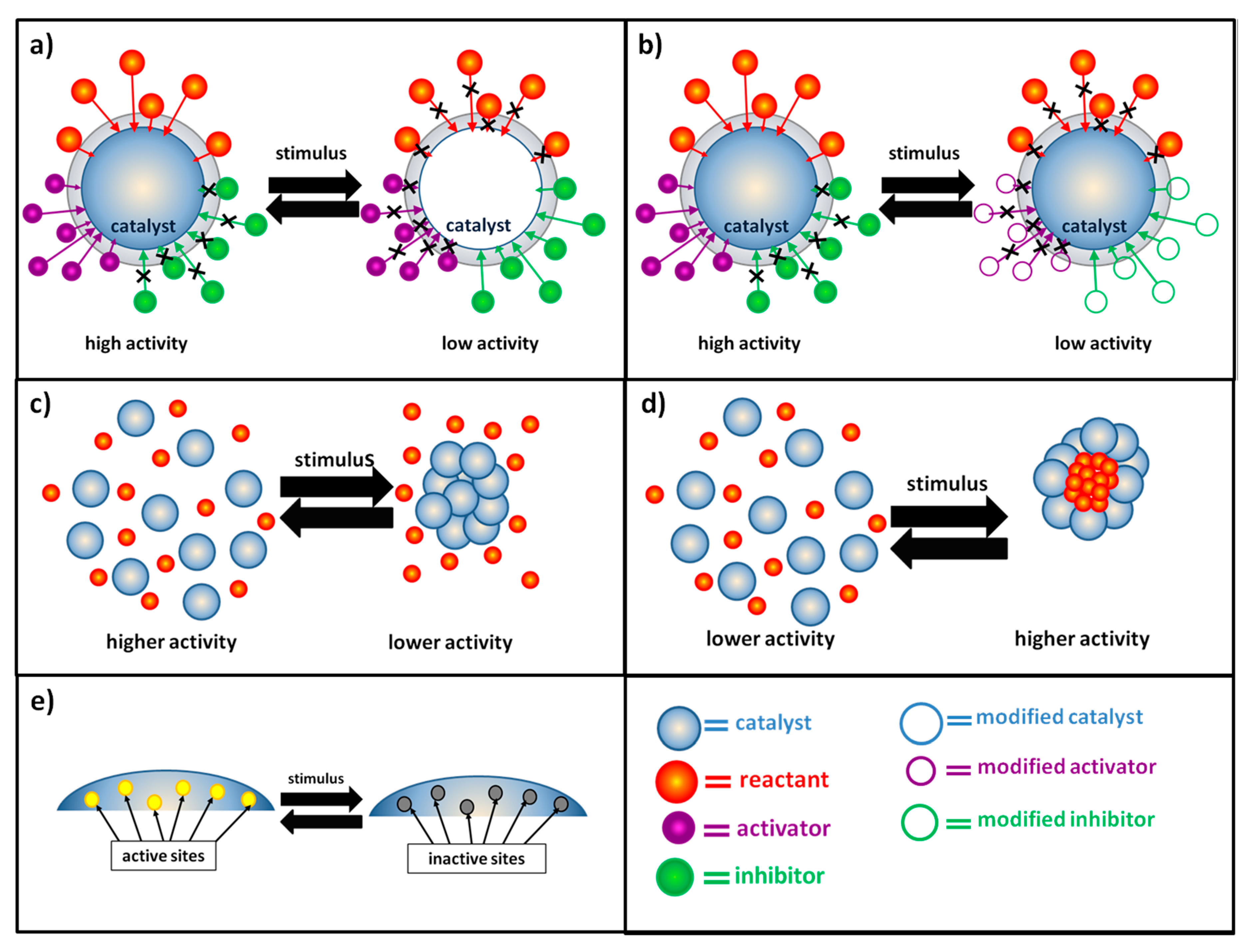
Catalysts Free FullText Switchable StimuliResponsive Heterogeneous Catalysis
Catalyst Examples When heated by itself, a sugar cube (sucrose) melts at 185°c. When heated by itself, a sugar cube (sucrose) melts at 185°c. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Some common examples of catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. A catalyst is a material that speeds up chemical reactions. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. How to burn a sugar cube. Also, discover how catalysts can be used figuratively to describe social or personal changes.
From
Catalyst Examples Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. Some common examples of catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. How to burn a sugar cube. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface. Catalyst Examples.
From
Catalyst Examples An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. A catalyst is a material that speeds up chemical reactions. When heated by itself, a sugar cube (sucrose) melts at. Catalyst Examples.
From
Catalyst Examples An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Some common examples of catalysis. Catalyst, in chemistry, any. Catalyst Examples.
From
Catalyst Examples An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn how catalysts work, why they are important and what. Catalyst Examples.
From
Catalyst Examples A catalyst is a material that speeds up chemical reactions. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal,. Catalyst Examples.
From www.slideshare.net
Catalysis Catalyst Examples An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a material that speeds up chemical reactions. Enzymes are naturally. Catalyst Examples.
From www.slideshare.net
Catalyst Catalyst Examples When heated by itself, a sugar cube (sucrose) melts at 185°c. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Also, discover how catalysts can be used figuratively to describe social or personal changes. How to burn a sugar cube. Catalyst, in chemistry, any substance. Catalyst Examples.
From
Catalyst Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. Enzymes are. Catalyst Examples.
From
Catalyst Examples A catalyst is a material that speeds up chemical reactions. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. How to burn a sugar cube. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids.. Catalyst Examples.
From
Catalyst Examples Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. A catalyst is a material that speeds up chemical reactions. Some common examples of catalysis. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes. Catalyst Examples.
From ar.inspiredpencil.com
Catalyst Examples For Kids Catalyst Examples Some common examples of catalysis. A catalyst is a material that speeds up chemical reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Also, discover how catalysts can be used figuratively to describe social or personal changes. An example of heterogeneous catalysis is the. Catalyst Examples.
From ar.inspiredpencil.com
Catalyst Examples In Everyday Life Catalyst Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a material that speeds up chemical reactions. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Catalyst Examples.
From
Catalyst Examples Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. A catalyst is a material that speeds up chemical. Catalyst Examples.
From www.slideshare.net
Biology 2.4 Catalyst Examples Also, discover how catalysts can be used figuratively to describe social or personal changes. How to burn a sugar cube. When heated by itself, a sugar cube (sucrose) melts at 185°c. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Catalyst, in chemistry, any substance that increases the rate of a reaction. Catalyst Examples.
From
Catalyst Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a material that speeds up chemical reactions. Some common examples of catalysis. Learn how catalysts work, why. Catalyst Examples.
From
Catalyst Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Also, discover how catalysts can be used figuratively to describe social or personal changes. How to burn a sugar cube. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. This page looks at the. Catalyst Examples.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2823635 Catalyst Examples Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. When heated by itself, a sugar cube (sucrose) melts at 185°c. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. This page looks at the the different types of catalyst (heterogeneous and homogeneous). Catalyst Examples.
From
Catalyst Examples Also, discover how catalysts can be used figuratively to describe social or personal changes. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. This page looks at. Catalyst Examples.
From
Catalyst Examples An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. A catalyst is a material that speeds up chemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.. Catalyst Examples.
From
Catalyst Examples When heated by itself, a sugar cube (sucrose) melts at 185°c. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. How to burn a sugar cube. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. Enzymes. Catalyst Examples.
From
Catalyst Examples Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Also, discover how catalysts can be used figuratively to describe social or personal changes. A catalyst is a material that. Catalyst Examples.
From
Catalyst Examples How to burn a sugar cube. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Also, discover how catalysts can be used. Catalyst Examples.
From
Catalyst Examples How to burn a sugar cube. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Some common examples of catalysis. When heated by itself, a sugar cube (sucrose) melts at 185°c. A catalyst is a material. Catalyst Examples.
From
Catalyst Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. When heated by itself, a sugar cube (sucrose) melts at 185°c. A catalyst is a material that speeds up chemical reactions. Learn how catalysts work,. Catalyst Examples.
From
Catalyst Examples Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal,. Catalyst Examples.
From
Catalyst Examples Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. When heated by itself, a sugar cube (sucrose) melts at 185°c. Also, discover how catalysts can be used figuratively to describe social or personal changes. How to burn a sugar cube. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. A. Catalyst Examples.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Examples Some common examples of catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Learn how catalysts work, why they are important and what examples of catalysts are used in. Catalyst Examples.
From
Catalyst Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. When heated by itself, a sugar cube (sucrose) melts at 185°c. A catalyst is a material that speeds up chemical reactions. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. How to burn a sugar cube.. Catalyst Examples.
From slideplayer.com
ppt download Catalyst Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Also, discover how catalysts can be used figuratively to describe social or personal changes. Some common examples of catalysis. Learn how catalysts work, why they are important and what examples of catalysts are used in industry. Catalyst Examples.
From
Catalyst Examples Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Some common examples of catalysis. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Learn how catalysts. Catalyst Examples.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Examples How to burn a sugar cube. Some common examples of catalysis. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. A catalyst is a material that speeds up chemical reactions. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Catalyst, in chemistry,. Catalyst Examples.
From www.youtube.com
Catalyst and Inhibitors YouTube Catalyst Examples Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. An. Catalyst Examples.
From
Catalyst Examples Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. A catalyst is a material that speeds up chemical reactions. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni,. Catalyst Examples.
From
Catalyst Examples Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. How to burn a sugar cube. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. An example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such. Catalyst Examples.
From
Catalyst Examples When heated by itself, a sugar cube (sucrose) melts at 185°c. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Learn how catalysts work, why they are important and what examples of catalysts are used in industry and nature. A catalyst is a material that speeds up chemical reactions. Also, discover how. Catalyst Examples.