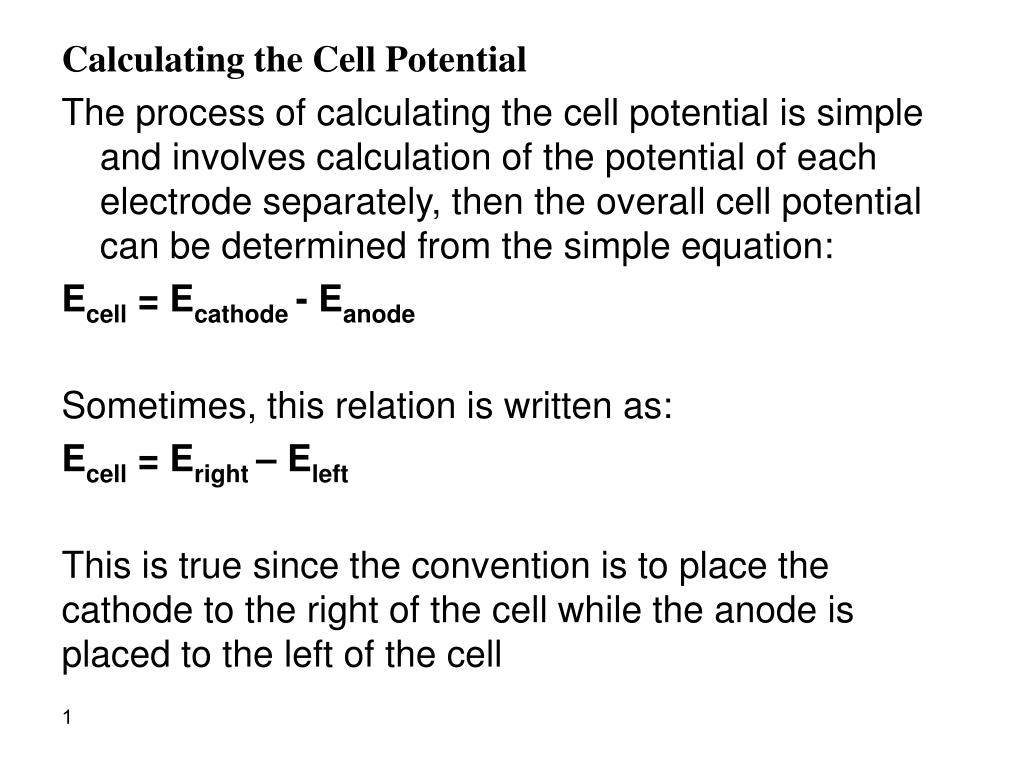
Standard Potential Cell Reaction . The potential difference is caused by the ability of. Describe and relate the definitions of electrode and cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential for a redox reaction (e cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The standard cell potential (e cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell.
from www.slideserve.com
Describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The standard cell potential (e cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Interpret electrode potentials in terms of relative oxidant and reductant.
PPT Calculating the Cell Potential PowerPoint Presentation, free download ID4347397
Standard Potential Cell Reaction The standard cell potential for a redox reaction (e cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. The standard cell potential (e cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential for a redox reaction (e cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; The potential difference is caused by the ability of.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Describe and relate the definitions of electrode and cell potentials.. Standard Potential Cell Reaction.
From www.chegg.com
Solved The standard cell potential, E°, for the reaction Standard Potential Cell Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. The potential difference is caused by the ability of. Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential. Standard Potential Cell Reaction.
From www.youtube.com
Standard Cell Potentials YouTube Standard Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. The potential difference is caused by the ability of. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \ (e_. Standard Potential Cell Reaction.
From mccord.cm.utexas.edu
Standard Potential Standard Potential Cell Reaction This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Describe and relate the definitions of electrode and cell potentials. The potential. Standard Potential Cell Reaction.
From www.chegg.com
Solved 5. Calculate the standard cell potential for the Standard Potential Cell Reaction The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. The standard cell potential (e cell) is measured at 298 k and all components in their standard states: 42 rows calculate the standard cell potential of a voltaic cell. Standard Potential Cell Reaction.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Potential Cell Reaction The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e cell) is measured at 298 k and all components in their standard states: We can calculate the standard potential for. Standard Potential Cell Reaction.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation, free download ID5480109 Standard Potential Cell Reaction This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in. Standard Potential Cell Reaction.
From mungfali.com
Standard Cell Potential Equation Standard Potential Cell Reaction 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: We can calculate the standard potential for any electrochemical cell from the. Standard Potential Cell Reaction.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Potential Cell Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. This is also known as the standard cell potential (e cell ) the standard cell. Standard Potential Cell Reaction.
From www.youtube.com
Calculating Standard Cell Potential YouTube Standard Potential Cell Reaction 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Interpret electrode potentials in terms of relative oxidant and reductant. We can calculate the standard potential for any electrochemical cell. Standard Potential Cell Reaction.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Potential Cell Reaction The standard cell potential (e cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the. Standard Potential Cell Reaction.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Standard Cell Potential Standard Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell.. Standard Potential Cell Reaction.
From byjus.com
Variation of Cell Potential in ZnCu Cell Chemistry Practicals Class 12, Experiment , Theory Standard Potential Cell Reaction The potential difference is caused by the ability of. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of. Standard Potential Cell Reaction.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Potential Cell Reaction The potential difference is caused by the ability of. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential for a redox reaction (e cell) is a measure of the. Standard Potential Cell Reaction.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. The standard cell potential (e cell) is measured at 298 k and all components in their standard states: The cell potential, \ (e_ {cell}\), is the measure of the potential difference between. Standard Potential Cell Reaction.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Potential Cell Reaction The standard cell potential (e cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The standard cell potential for a redox reaction (e cell). Standard Potential Cell Reaction.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant. We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods:. Standard Potential Cell Reaction.
From www.vrogue.co
Standard Reduction Potentials Redox Reactions And Ele vrogue.co Standard Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The potential difference is caused by the ability of. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The cell potential, \ (e_ {cell}\), is the measure. Standard Potential Cell Reaction.
From chemistrynotes.com
Cell Potential Calculations and Line Notation Standard Potential Cell Reaction The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Interpret electrode potentials in terms of relative oxidant and reductant. This is also. Standard Potential Cell Reaction.
From www.slideserve.com
PPT Calculating the Cell Potential PowerPoint Presentation, free download ID4347397 Standard Potential Cell Reaction 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential for a redox reaction (e cell) is a measure of the tendency of reactants in their standard states to. Standard Potential Cell Reaction.
From www.slideserve.com
PPT Cell Potential and Nernst Equation PowerPoint Presentation, free download ID3222476 Standard Potential Cell Reaction We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. The standard cell potential (e cell) is measured at 298 k and all components in their standard states: The potential difference is caused by the ability of. This is also known as the standard cell potential (e cell ) the standard cell. Standard Potential Cell Reaction.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Potential Cell Reaction The potential difference is caused by the ability of. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The cell potential, \ (e_ {cell}\), is the measure. Standard Potential Cell Reaction.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium/Silver Galvanic Cell Nagwa Standard Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability. Standard Potential Cell Reaction.
From www.chegg.com
Solved 5. What is the standard cell potential (E cel) for Standard Potential Cell Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential for a redox reaction (e cell) is a measure of the. Standard Potential Cell Reaction.
From www.chegg.com
Solved Calculate the standard cell potential for each of the Standard Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. 42 rows calculate the standard cell potential of a voltaic. Standard Potential Cell Reaction.
From www.chegg.com
Solved Calculate the standard cell potential for a voltaic Standard Potential Cell Reaction We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential, \ (e_ {cell}\), is the. Standard Potential Cell Reaction.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Standard Potential Cell Reaction The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. We can calculate the standard potential for any electrochemical cell from the standard potentials of. Standard Potential Cell Reaction.
From www.youtube.com
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems Standard Potential Cell Reaction The standard cell potential (e cell) is measured at 298 k and all components in their standard states: The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two. Standard Potential Cell Reaction.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Potential Cell Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The potential difference. Standard Potential Cell Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Potential Cell Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential for a redox reaction (e cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells. Standard Potential Cell Reaction.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID2955286 Standard Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of. Describe and relate the definitions of electrode and cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The. Standard Potential Cell Reaction.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID5581588 Standard Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant. We can calculate the standard potential for any electrochemical cell from the standard potentials of the two half. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid. Standard Potential Cell Reaction.
From pveducation.org
Standard Potential PVEducation Standard Potential Cell Reaction This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. Describe and relate the definitions of electrode. Standard Potential Cell Reaction.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation, free download ID5480109 Standard Potential Cell Reaction 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The standard cell. Standard Potential Cell Reaction.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential, \ (e_ {cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. We can calculate the standard potential for any electrochemical. Standard Potential Cell Reaction.