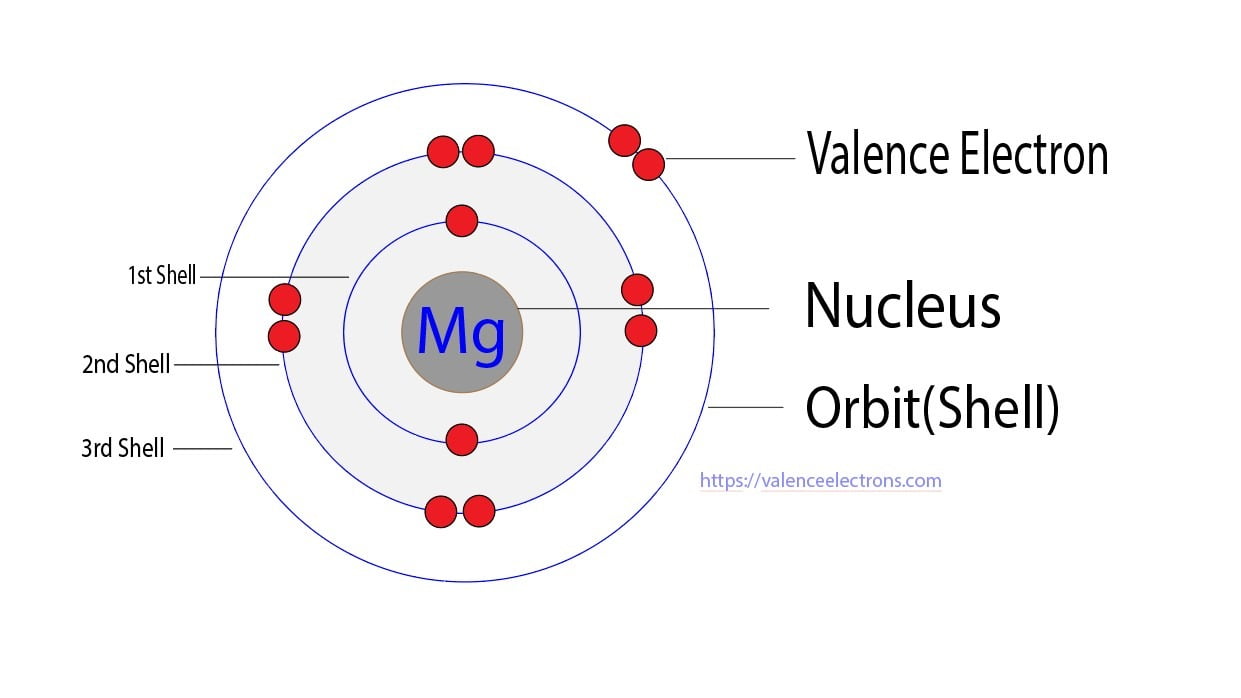
Electrons Magnesium Ion Have . Magnesium has 12 electrons arranged in three energy. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. Possible oxidation states are +2. electron configuration of magnesium is [ne] 3s2. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².
from valenceelectrons.com
Magnesium has 12 electrons arranged in three energy. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. Possible oxidation states are +2. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². electron configuration of magnesium is [ne] 3s2.
How to Write the Electron Configuration for Magnesium (Mg)?
Electrons Magnesium Ion Have electron configuration of magnesium is [ne] 3s2. electron configuration of magnesium is [ne] 3s2. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Electrons Magnesium Ion Have Magnesium has 12 electrons arranged in three energy. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. Possible oxidation states are +2. electron configuration of magnesium is [ne] 3s2. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the 2+ charge indicates that you have lost. Electrons Magnesium Ion Have.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Electrons Magnesium Ion Have Possible oxidation states are +2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Magnesium has 12 electrons. Electrons Magnesium Ion Have.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Electrons Magnesium Ion Have the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Electrons Magnesium Ion Have.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electrons Magnesium Ion Have electron configuration of magnesium is [ne] 3s2. Possible oxidation states are +2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. in order to write the mg electron configuration we first need to know the number of. Electrons Magnesium Ion Have.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Electrons Magnesium Ion Have The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. electron configuration of magnesium is [ne] 3s2. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states. Electrons Magnesium Ion Have.
From ar.inspiredpencil.com
Magnesium Atom Structure Electrons Magnesium Ion Have the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. electron configuration of magnesium is [ne] 3s2.. Electrons Magnesium Ion Have.
From gioyknlly.blob.core.windows.net
Magnesium Valence Electrons Ion at Elena Epps blog Electrons Magnesium Ion Have Magnesium has 12 electrons arranged in three energy. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states are +2. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. electron configuration of magnesium is [ne] 3s2. magnesium is the eighth most abundant. Electrons Magnesium Ion Have.
From www.shutterstock.com
Diagram Representation Element Magnesium Neutrons Protons Stock Vector Electrons Magnesium Ion Have Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the 2+ charge indicates that you. Electrons Magnesium Ion Have.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Electrons Magnesium Ion Have The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states are +2. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the 2+ charge indicates that you have lost two electrons, which means that we'd. Electrons Magnesium Ion Have.
From slideplayer.com
SOL Review Game ppt download Electrons Magnesium Ion Have Magnesium has 12 electrons arranged in three energy. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. Possible oxidation states are +2. magnesium is the eighth. Electrons Magnesium Ion Have.
From brainly.com
⚗️What is the electron configuration for the Magnesium ion? Electrons Magnesium Ion Have magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. Magnesium has 12 electrons arranged in three energy. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states are +2. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg. Electrons Magnesium Ion Have.
From www.slideserve.com
PPT How many valence electrons does magnesium have? PowerPoint Electrons Magnesium Ion Have The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg electron configuration we first need to know the number of electrons for the. Electrons Magnesium Ion Have.
From www.animalia-life.club
Magnesium Electron Configuration Electrons Magnesium Ion Have the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. electron configuration of magnesium is [ne] 3s2. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. in. Electrons Magnesium Ion Have.
From gioxkuzlt.blob.core.windows.net
Magnesium Ion Have Electrons at Barbara Finn blog Electrons Magnesium Ion Have Magnesium has 12 electrons arranged in three energy. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states. Electrons Magnesium Ion Have.
From cartoondealer.com
Magnesium Atom Bohr Model Cartoon Vector 267662111 Electrons Magnesium Ion Have in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Possible oxidation states are +2. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the 2+ charge indicates that you have lost two electrons, which means that. Electrons Magnesium Ion Have.
From www.numerade.com
SOLVED What happens when the compound Mgo is formed? (5 points) Oxygen Electrons Magnesium Ion Have Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. electron configuration of magnesium is [ne]. Electrons Magnesium Ion Have.
From gioyknlly.blob.core.windows.net
Magnesium Valence Electrons Ion at Elena Epps blog Electrons Magnesium Ion Have Possible oxidation states are +2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the 2+ charge indicates that you have lost two electrons, which. Electrons Magnesium Ion Have.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Electrons Magnesium Ion Have The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. Magnesium has 12 electrons arranged in three energy. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. Possible oxidation states. Electrons Magnesium Ion Have.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Electrons Magnesium Ion Have in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Magnesium has 12 electrons arranged in three energy. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. Possible oxidation states are +2. the electron configuration of magnesium. Electrons Magnesium Ion Have.
From enginedatanichered.z21.web.core.windows.net
Magnesium Atom Diagram Electrons Magnesium Ion Have electron configuration of magnesium is [ne] 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the 2+ charge indicates that you have lost two electrons, which means that we'd. Electrons Magnesium Ion Have.
From www.animalia-life.club
Magnesium Electron Configuration Electrons Magnesium Ion Have magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². electron configuration of magnesium is [ne] 3s2. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Possible. Electrons Magnesium Ion Have.
From hxeotzdbq.blob.core.windows.net
Magnesium Ion Number Of Protons at Beverly Gilmore blog Electrons Magnesium Ion Have magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. Possible oxidation states are +2. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12. Electrons Magnesium Ion Have.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Electrons Magnesium Ion Have The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the 2+ charge indicates that you have lost two electrons, which means that we'd. Electrons Magnesium Ion Have.
From exycocyow.blob.core.windows.net
Magnesium V Ion at Jacqueline Sneed blog Electrons Magnesium Ion Have the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy. magnesium is the eighth most abundant element in the earth’s. Electrons Magnesium Ion Have.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Electrons Magnesium Ion Have electron configuration of magnesium is [ne] 3s2. Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the electron configuration of magnesium is. Electrons Magnesium Ion Have.
From zaiden-has-parks.blogspot.com
How Many Valence Electrons Does a Neutral Magnesium Atom Have Zaiden Electrons Magnesium Ion Have the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy. in order to write the mg electron configuration we first need to know the number of electrons for the mg atom.. Electrons Magnesium Ion Have.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Electrons Magnesium Ion Have Magnesium has 12 electrons arranged in three energy. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². electron configuration of magnesium is [ne] 3s2. in order. Electrons Magnesium Ion Have.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Electrons Magnesium Ion Have the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy. electron configuration of magnesium is [ne] 3s2. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from. Electrons Magnesium Ion Have.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Electrons Magnesium Ion Have magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. electron configuration of magnesium is [ne] 3s2.. Electrons Magnesium Ion Have.
From www.dreamstime.com
Atom of Magnesium with Detailed Core and 12 Electrons on White with Electrons Magnesium Ion Have Possible oxidation states are +2. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. electron configuration of magnesium is [ne] 3s2. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. in order to write the mg electron. Electrons Magnesium Ion Have.
From www.flexiprep.com
NCERT Class 9 Science Solutions Chapter 3 Atoms and Molecules Part 9 Electrons Magnesium Ion Have magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg electron configuration we first need to know the number of electrons for the. Electrons Magnesium Ion Have.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Electrons Magnesium Ion Have magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. Possible oxidation states are +2. in order to write the mg electron configuration we first need to know the number. Electrons Magnesium Ion Have.
From awesomehome.co
Magnesium Periodic Table Electrons Awesome Home Electrons Magnesium Ion Have electron configuration of magnesium is [ne] 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states are +2. the 2+ charge indicates that you have lost two electrons, which means that we'd subtract two from the original. Magnesium has 12 electrons arranged in three energy. the electron configuration of magnesium is 1s² 2s². Electrons Magnesium Ion Have.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Electrons Magnesium Ion Have in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Possible oxidation states are +2. Magnesium has 12 electrons arranged in three energy. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². the 2+ charge. Electrons Magnesium Ion Have.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Electrons Magnesium Ion Have Possible oxidation states are +2. magnesium is the eighth most abundant element in the earth’s crust, but does not occur uncombined in nature. the electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². in order to write the mg electron configuration we first need to know the number of electrons for the mg atom. electron configuration. Electrons Magnesium Ion Have.