Bromide Sodium Hydroxide . There is one cobalt ion. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Record the colour of any. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Dissolve a small quantity of the substance in water. The balanced equation will be. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Enter an equation of an ionic chemical equation and press the balance button. Place about 5cm 3 of the solution into a test tube. Add a few drops of sodium hydroxide solution. Then, identify the anion and write down its.
from www.shutterstock.com
Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Record the colour of any. The balanced equation will be. Place about 5cm 3 of the solution into a test tube. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Add a few drops of sodium hydroxide solution. There is one cobalt ion. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Dissolve a small quantity of the substance in water. Then, identify the anion and write down its.
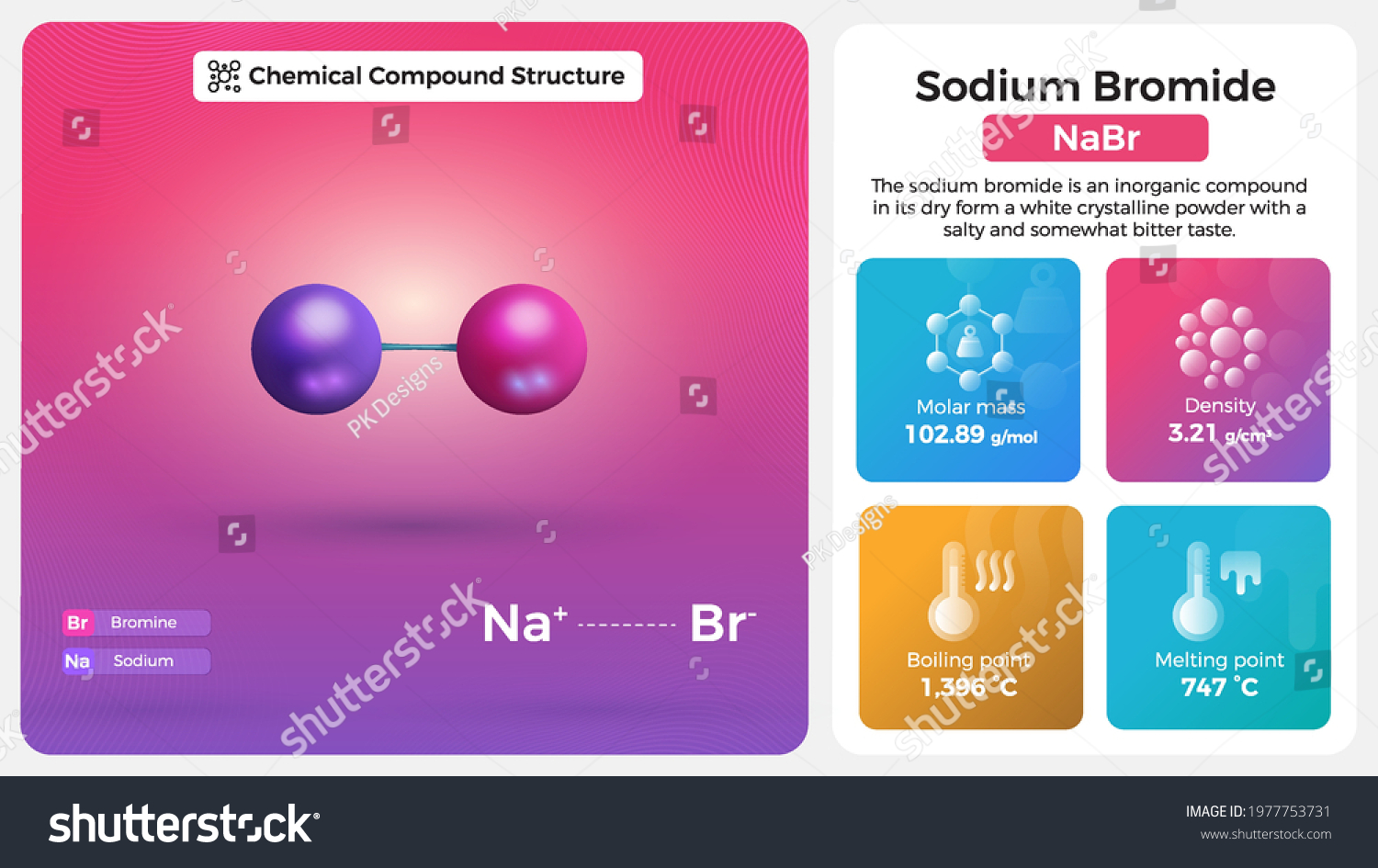
Sodium Bromide Properties Chemical Compound Structure Stock Vector
Bromide Sodium Hydroxide The balanced equation will be. Then, identify the anion and write down its. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Place about 5cm 3 of the solution into a test tube. Enter an equation of an ionic chemical equation and press the balance button. Record the colour of any. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Add a few drops of sodium hydroxide solution. Dissolve a small quantity of the substance in water. There is one cobalt ion. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The balanced equation will be.
From www.numerade.com
SOLVED Aqueous hydrobromic acid HBr will react with solid sodium Bromide Sodium Hydroxide Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Dissolve a small quantity of the substance in water. There is one cobalt ion. Iron(iii) ions pair up with hydroxide ions. Bromide Sodium Hydroxide.
From testbook.com
Sodium Bromide Learn Definition, Properties, Structure & Uses Bromide Sodium Hydroxide This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three. Bromide Sodium Hydroxide.
From www.shutterstock.com
Sodium Salts (Set 2) Sodium Fluoride, Chloride, Bromide, Iodide Bromide Sodium Hydroxide To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The balanced equation will be. Dissolve a small quantity of the substance in water. Record the colour of any. Add a few drops of sodium hydroxide solution. Place about 5cm 3 of the solution into a test tube. Then, identify the. Bromide Sodium Hydroxide.
From hamptonresearch.com
Hampton Research Bromide Sodium Hydroxide The balanced equation will be. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Record the colour of any. There is one cobalt ion. Bromine and sodium hydroxide. Bromide Sodium Hydroxide.
From www.numerade.com
SOLVED Aqueous hydrobromic acid (HBr) reacts with solid sodium Bromide Sodium Hydroxide Place about 5cm 3 of the solution into a test tube. Then, identify the anion and write down its. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). There is one cobalt ion. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v). Bromide Sodium Hydroxide.
From ask.modifiyegaraj.com
What Best Describes The Bromide Ion That Forms Asking List Bromide Sodium Hydroxide The balanced equation will be. Then, identify the anion and write down its. Enter an equation of an ionic chemical equation and press the balance button. Add a few drops of sodium hydroxide solution. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. This page looks at the. Bromide Sodium Hydroxide.
From www.coursehero.com
[Solved] Aqueous hydrobromic acid HBr reacts with solid sodium Bromide Sodium Hydroxide The balanced equation will be. Record the colour of any. Enter an equation of an ionic chemical equation and press the balance button. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Dissolve a small quantity of the substance in water. To find the formula of an ionic. Bromide Sodium Hydroxide.
From www.universalmedicalinc.com
IBI IB40075 Ethidium Bromide Solution 10mL Bromide Sodium Hydroxide Enter an equation of an ionic chemical equation and press the balance button. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Place about 5cm 3 of the solution into a test tube. Add a few drops of sodium hydroxide solution. Iron(iii) ions pair up with hydroxide ions. Bromide Sodium Hydroxide.
From www.firsthope.co.in
Methantheline Bromide Chemical Structure, Mechanism of Action, Uses Bromide Sodium Hydroxide Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. The balanced equation will be. Then, identify the anion and write down its. Dissolve a small quantity of the substance in. Bromide Sodium Hydroxide.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Bromide Sodium Hydroxide Dissolve a small quantity of the substance in water. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Record the colour of any. Bromine and sodium hydroxide solution. Bromide Sodium Hydroxide.
From www.flinnsci.ca
Sodium Bromide, Reagent, 100 g Flinn Scientific Bromide Sodium Hydroxide Dissolve a small quantity of the substance in water. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. The balanced equation will be. Bromine and sodium hydroxide solution with bromine, the formation. Bromide Sodium Hydroxide.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromide Sodium Hydroxide Then, identify the anion and write down its. The balanced equation will be. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. There is one cobalt ion. Add a few drops of sodium hydroxide solution. Record the colour of any. Enter an equation of an ionic chemical equation and press the. Bromide Sodium Hydroxide.
From www.numerade.com
SOLVED Aqueous hydrobromic acid (HBr) reacts with solid sodium Bromide Sodium Hydroxide Then, identify the anion and write down its. Add a few drops of sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. To find the formula of an ionic compound, first identify. Bromide Sodium Hydroxide.
From askfilo.com
Reaction of methyl bromide with aqueous sodium hydroxide involves Filo Bromide Sodium Hydroxide Add a few drops of sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Bromine and. Bromide Sodium Hydroxide.
From www.youtube.com
Sodium bromide YouTube Bromide Sodium Hydroxide Record the colour of any. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. The balanced equation will be. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide,. Bromide Sodium Hydroxide.
From www.bhphotovideo.com
Photographers' Formulary Sodium Bromide 1 Lb. 101180 1LB B&H Bromide Sodium Hydroxide There is one cobalt ion. Dissolve a small quantity of the substance in water. Then, identify the anion and write down its. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will. Bromide Sodium Hydroxide.
From www.numerade.com
SOLVED Aqueous hydrobromic acid (HBr) reacts with solid sodium Bromide Sodium Hydroxide Record the colour of any. There is one cobalt ion. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Place about 5cm 3 of the solution into a test tube. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a. Bromide Sodium Hydroxide.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide Sodium Hydroxide Dissolve a small quantity of the substance in water. There is one cobalt ion. Add a few drops of sodium hydroxide solution. Record the colour of any. Place about 5cm 3 of the solution into a test tube. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. To. Bromide Sodium Hydroxide.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromide Sodium Hydroxide This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Then, identify the anion and write down its. Place about 5cm 3 of the solution into a test tube. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −).. Bromide Sodium Hydroxide.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromide Sodium Hydroxide Enter an equation of an ionic chemical equation and press the balance button. Then, identify the anion and write down its. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or.. Bromide Sodium Hydroxide.
From www.numerade.com
SOLVED write the balanced equation for each of the reactions 7 Sodium Bromide Sodium Hydroxide Dissolve a small quantity of the substance in water. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The balanced equation will be. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Record the colour of. Bromide Sodium Hydroxide.
From www.chemistrylearner.com
Sodium Bromate Facts, Formula, Properties, Uses, Safety Data Bromide Sodium Hydroxide Dissolve a small quantity of the substance in water. Then, identify the anion and write down its. Place about 5cm 3 of the solution into a test tube. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. There is one cobalt ion. Record the colour of any. Iron(iii) ions pair up. Bromide Sodium Hydroxide.
From www.nanochemazone.com
Sodium Bromide Powder Low Price 1 highly pure Nanochemazone Bromide Sodium Hydroxide Then, identify the anion and write down its. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Enter an equation of an ionic chemical equation and press the balance button. Dissolve a small quantity of the substance in water. Add a few drops of sodium hydroxide solution. Iron(iii) ions pair. Bromide Sodium Hydroxide.
From www.chegg.com
Solved Aqueous hydrobromic acid will react with solid sodium Bromide Sodium Hydroxide There is one cobalt ion. Enter an equation of an ionic chemical equation and press the balance button. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Dissolve a small quantity of the substance in water. To find the formula of an ionic compound, first identify. Bromide Sodium Hydroxide.
From www.coursehero.com
[Solved] When benzyl bromide reacts with aqueous sodium hydroxide, an Bromide Sodium Hydroxide Enter an equation of an ionic chemical equation and press the balance button. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Add a few drops of sodium hydroxide solution. The balanced equation will be. There is one cobalt ion. Dissolve a small quantity of the substance in. Bromide Sodium Hydroxide.
From sielc.com
Vinyl bromide SIELC Technologies Bromide Sodium Hydroxide Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Dissolve a small quantity of the substance in water. Enter an equation of an ionic chemical equation and press the. Bromide Sodium Hydroxide.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Bromide Sodium Hydroxide Add a few drops of sodium hydroxide solution. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. There is one cobalt ion. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. The balanced equation will be. Iron(iii) ions pair. Bromide Sodium Hydroxide.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Bromide Sodium Hydroxide Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. The balanced equation will be. Dissolve a small quantity of the substance in water. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Enter an equation of an ionic. Bromide Sodium Hydroxide.
From nsilabsolutions.com
Bromide CRM IS019 NSI Lab Solutions Bromide Sodium Hydroxide Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −). Enter an equation of an ionic chemical equation and press the balance button. Then, identify the anion and write down its. There is one cobalt ion. To find the formula of an ionic compound, first identify the. Bromide Sodium Hydroxide.
From www.toptionchem.com
China Sodium Bromide Manufacture and Factory Toption Bromide Sodium Hydroxide To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe 3 + balances the charge of three oh −).. Bromide Sodium Hydroxide.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide Sodium Hydroxide Enter an equation of an ionic chemical equation and press the balance button. Record the colour of any. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Add a few. Bromide Sodium Hydroxide.
From www.indiamart.com
Sodium Bromide 45, Bromide salt of sodium, NaBr, 7647156, Sedoneural Bromide Sodium Hydroxide Dissolve a small quantity of the substance in water. There is one cobalt ion. Place about 5cm 3 of the solution into a test tube. Enter an equation of an ionic chemical equation and press the balance button. Then, identify the anion and write down its. Iron(iii) ions pair up with hydroxide ions forming iron(iii) hydroxide, fe(oh) 3 (one fe. Bromide Sodium Hydroxide.
From www.numerade.com
SOLVED A student writes the following incorrect chemical equation for Bromide Sodium Hydroxide To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Dissolve a small quantity of the substance in water. Enter an equation of an ionic chemical equation and press the balance button. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature,. Bromide Sodium Hydroxide.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide Sodium Hydroxide There is one cobalt ion. Then, identify the anion and write down its. Dissolve a small quantity of the substance in water. Place about 5cm 3 of the solution into a test tube. This page looks at the reactions between halogenoalkanes (haloalkanes or alkyl halides) and hydroxide ions from sodium or. Enter an equation of an ionic chemical equation and. Bromide Sodium Hydroxide.
From www.indiamart.com
BioTech Grade Powder Sodium Bromide for Petroleum Industry, for Bromide Sodium Hydroxide Then, identify the anion and write down its. Bromine and sodium hydroxide solution with bromine, the formation of the sodium bromate(v) happens at a much lower temperature, down to. Dissolve a small quantity of the substance in water. Enter an equation of an ionic chemical equation and press the balance button. Place about 5cm 3 of the solution into a. Bromide Sodium Hydroxide.