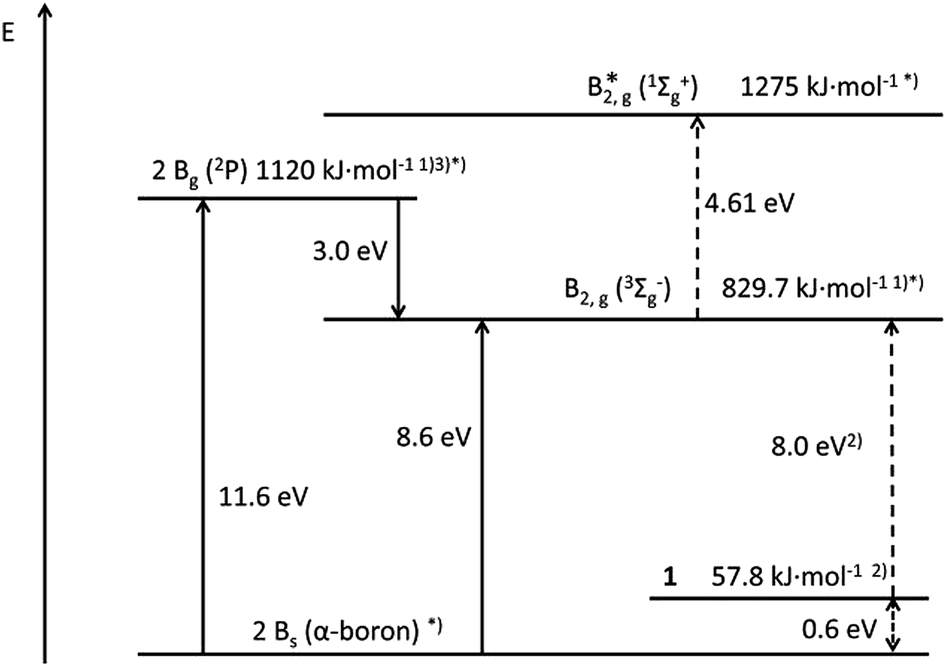
Is Boron Stable Or Reactive . Amorphous form of boron is reactive. It makes stable covalent bonds with other compounds and does not forms. Boron is a poor room temperature. Elemental boron is a semimetal that is remarkably unreactive. Boron hydrides are used to synthesize organic compounds. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron forms unique and intricate structures that contain multicenter bonds, in. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Although opaque to visible light, boron can transmit portions of infrared light. In crystalline form, boron is quite unreactive. Reactivity towards acids and alkalis:
from pubs.rsc.org
Reactivity towards acids and alkalis: Although opaque to visible light, boron can transmit portions of infrared light. Boron does not generally make ionic bonds, it forms stable covalent bonds. It makes stable covalent bonds with other compounds and does not forms. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Boron is a poor room temperature. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). In crystalline form, boron is quite unreactive. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron forms unique and intricate structures that contain multicenter bonds, in.
The boronboron triple bond? A thermodynamic and force field based
Is Boron Stable Or Reactive Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron hydrides are used to synthesize organic compounds. Boron is a poor room temperature. Reactivity towards acids and alkalis: Amorphous form of boron is reactive. It makes stable covalent bonds with other compounds and does not forms. In crystalline form, boron is quite unreactive. Boron does not generally make ionic bonds, it forms stable covalent bonds. Although opaque to visible light, boron can transmit portions of infrared light. Boron forms unique and intricate structures that contain multicenter bonds, in. Elemental boron is a semimetal that is remarkably unreactive. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures.
From pubs.acs.org
Relative Reactivity of Stable Ligated Boranes and a Borohydride Salt in Is Boron Stable Or Reactive Elemental boron is a semimetal that is remarkably unreactive. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron forms unique and intricate structures that contain multicenter bonds, in. Amorphous form of boron is reactive. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. It makes stable covalent bonds with other compounds. Is Boron Stable Or Reactive.
From www.researchgate.net
Experimentally determined stability fields for boron minerals in the Is Boron Stable Or Reactive Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. In crystalline form, boron is quite unreactive. Amorphous form of boron is reactive. It makes stable covalent bonds. Is Boron Stable Or Reactive.
From medium.com
What is Boron? Periodic Table Elements Is Boron Stable Or Reactive Although opaque to visible light, boron can transmit portions of infrared light. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). In crystalline form, boron is quite unreactive. It makes stable. Is Boron Stable Or Reactive.
From www.researchgate.net
(A) Boronmasking strategy. (B) sBond metathesis with (amide)BB(pin Is Boron Stable Or Reactive Although opaque to visible light, boron can transmit portions of infrared light. Reactivity towards acids and alkalis: Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Boron is a poor room temperature. Elemental boron is a semimetal that is remarkably unreactive. Boron forms unique and intricate structures that contain multicenter bonds, in. Boron hydrides are. Is Boron Stable Or Reactive.
From borates.today
Stable 2D Structures Of Boron Borates Today Is Boron Stable Or Reactive Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Reactivity towards acids and alkalis: In crystalline form, boron is quite unreactive. Although opaque to visible light, boron can transmit portions of infrared light. Boron is a poor room temperature. Elemental boron is a semimetal that is remarkably unreactive. Boron does not generally make ionic bonds,. Is Boron Stable Or Reactive.
From www.slideserve.com
PPT Boron (p102) PowerPoint Presentation, free download ID947998 Is Boron Stable Or Reactive Boron hydrides are used to synthesize organic compounds. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is a poor room temperature. Amorphous form of boron is reactive. Although opaque to visible light, boron can transmit portions of infrared light. Elemental boron is a semimetal that is remarkably unreactive. Boron forms unique. Is Boron Stable Or Reactive.
From www.pearson.com
All the stable isotopes of boron, carbon, nitrogen, oxygen, and f Is Boron Stable Or Reactive Although opaque to visible light, boron can transmit portions of infrared light. Amorphous form of boron is reactive. Boron hydrides are used to synthesize organic compounds. Boron is a poor room temperature. It makes stable covalent bonds with other compounds and does not forms. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). In crystalline form, boron. Is Boron Stable Or Reactive.
From www.researchgate.net
Boroncentered stable radical anion/radical cation pair. Reproduced Is Boron Stable Or Reactive Elemental boron is a semimetal that is remarkably unreactive. Reactivity towards acids and alkalis: Boron forms unique and intricate structures that contain multicenter bonds, in. It makes stable covalent bonds with other compounds and does not forms. Although opaque to visible light, boron can transmit portions of infrared light. Boron is unreactive when comes in contact with acids and alkalis. Is Boron Stable Or Reactive.
From leah4sci.com
Free Radicals Organic Chemistry Reactions, Resonance, Stability Is Boron Stable Or Reactive Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron hydrides are used to synthesize organic compounds. Amorphous form of boron is reactive. Reactivity towards acids and alkalis: Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron. Is Boron Stable Or Reactive.
From www.slideserve.com
PPT Boron (p102) PowerPoint Presentation ID947998 Is Boron Stable Or Reactive Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. It makes stable covalent bonds with. Is Boron Stable Or Reactive.
From manuallistferraro.z13.web.core.windows.net
Electron Structure Of Boron Is Boron Stable Or Reactive Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. In crystalline form, boron is quite unreactive. Boron forms unique and intricate structures that contain multicenter bonds, in. Amorphous form of boron is reactive. Although opaque to visible light, boron can transmit portions of infrared light. It makes stable covalent bonds with other compounds and does. Is Boron Stable Or Reactive.
From www.slideserve.com
PPT Main group III elements PowerPoint Presentation, free download Is Boron Stable Or Reactive It makes stable covalent bonds with other compounds and does not forms. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Boron forms unique and intricate structures that contain multicenter bonds, in. Amorphous form of boron is reactive. Although. Is Boron Stable Or Reactive.
From www.britannica.com
Boron Properties, Uses, & Facts Britannica Is Boron Stable Or Reactive Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Reactivity towards acids and alkalis: Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. In crystalline form, boron is quite unreactive. Boron hydrides are used to synthesize organic compounds. Amorphous form of boron is reactive. Boron does not generally make ionic. Is Boron Stable Or Reactive.
From byjus.com
stability order of +1 states of boron family Is Boron Stable Or Reactive Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. In crystalline form, boron is quite unreactive. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is a poor room temperature. It makes stable covalent bonds with other compounds and does not forms. Boron does not generally make. Is Boron Stable Or Reactive.
From www.slideserve.com
PPT Boron (p102) PowerPoint Presentation, free download ID947998 Is Boron Stable Or Reactive Elemental boron is a semimetal that is remarkably unreactive. Although opaque to visible light, boron can transmit portions of infrared light. Boron is a poor room temperature. It makes stable covalent bonds with other compounds and does not forms. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is unreactive when comes. Is Boron Stable Or Reactive.
From www.youtube.com
Boron has two stable isotopes, 10B (19) and 11B (81). Calculate Is Boron Stable Or Reactive Boron hydrides are used to synthesize organic compounds. Reactivity towards acids and alkalis: Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Amorphous form of boron is reactive. Boron forms unique and intricate structures that contain multicenter bonds, in. In crystalline. Is Boron Stable Or Reactive.
From pages.swcp.com
ON QUARKS, NUCLEI and BORON10 NEUTRON CAPTURE Is Boron Stable Or Reactive Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron hydrides are used to synthesize organic compounds. It makes stable covalent bonds with other compounds and does not forms. Boron forms unique and intricate structures that contain multicenter bonds, in. In crystalline form, boron is quite unreactive. Amorphous form of boron is reactive.. Is Boron Stable Or Reactive.
From www.youtube.com
Boron Stable Isotopes YouTube Is Boron Stable Or Reactive Boron forms unique and intricate structures that contain multicenter bonds, in. Amorphous form of boron is reactive. In crystalline form, boron is quite unreactive. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Elemental boron is a semimetal that is remarkably unreactive. Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron, chemical element. Is Boron Stable Or Reactive.
From www.researchgate.net
Stability of boron phases at 0 K. Enthalpies are shown relative to αB Is Boron Stable Or Reactive Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron is a poor room temperature. In crystalline form, boron is quite unreactive. Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron forms unique and intricate structures that contain multicenter bonds, in. Although opaque to visible light, boron can transmit portions of infrared light.. Is Boron Stable Or Reactive.
From www.slideserve.com
PPT Boron (p102) PowerPoint Presentation, free download ID947998 Is Boron Stable Or Reactive Boron is a poor room temperature. It makes stable covalent bonds with other compounds and does not forms. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. In crystalline form, boron is quite unreactive. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Reactivity towards acids and alkalis:. Is Boron Stable Or Reactive.
From pubs.rsc.org
The boronboron triple bond? A thermodynamic and force field based Is Boron Stable Or Reactive Boron forms unique and intricate structures that contain multicenter bonds, in. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Elemental boron is a semimetal that is remarkably unreactive. Boron does not generally make ionic bonds, it forms stable covalent bonds. In crystalline form, boron is quite unreactive. Boron tends to forms hydrides, the simplest. Is Boron Stable Or Reactive.
From chemistry.stackexchange.com
organic chemistry Why is this intermediate boranehydroperoxide Is Boron Stable Or Reactive Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron forms unique and intricate structures that contain multicenter bonds, in. Elemental boron is a semimetal that is remarkably unreactive. Amorphous form of boron is reactive. In crystalline form, boron is quite unreactive. Boron does not generally make ionic bonds, it forms stable covalent. Is Boron Stable Or Reactive.
From classfullcasandra.z21.web.core.windows.net
Reactivity Chart Periodic Table Is Boron Stable Or Reactive In crystalline form, boron is quite unreactive. Boron forms unique and intricate structures that contain multicenter bonds, in. Boron is a poor room temperature. Although opaque to visible light, boron can transmit portions of infrared light. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. It makes stable covalent bonds with other compounds and does. Is Boron Stable Or Reactive.
From www.youtube.com
Boron has two stable isotopes, B10 (19) and B11 (81). Calculate Is Boron Stable Or Reactive Elemental boron is a semimetal that is remarkably unreactive. Although opaque to visible light, boron can transmit portions of infrared light. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. It makes stable covalent bonds with other compounds and does not. Is Boron Stable Or Reactive.
From www.slideserve.com
PPT Boron (p102) PowerPoint Presentation, free download ID947998 Is Boron Stable Or Reactive Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. In crystalline form, boron is quite unreactive. Boron is a poor room temperature. Boron forms unique and intricate structures that contain multicenter bonds, in. Reactivity towards acids and alkalis: Amorphous form of boron is reactive. Elemental boron is a semimetal that is remarkably unreactive. Boron, chemical. Is Boron Stable Or Reactive.
From www.semanticscholar.org
Figure 1 from Boron as a powerful reductant synthesis of a stable Is Boron Stable Or Reactive Although opaque to visible light, boron can transmit portions of infrared light. Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron hydrides are used to synthesize organic compounds. Amorphous form of boron is reactive. In crystalline form, boron is quite unreactive. It makes stable covalent bonds with other compounds and does not forms. Boron is a. Is Boron Stable Or Reactive.
From pubs.acs.org
First Example of Stable 1,1Bimetalloalkenes of Boron and Zirconium Is Boron Stable Or Reactive Reactivity towards acids and alkalis: Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron hydrides are used to synthesize organic compounds. Boron forms unique and intricate structures that. Is Boron Stable Or Reactive.
From www.numerade.com
SOLVEDBoron, lithium, and nitrogen each have two stable isotopes. Use Is Boron Stable Or Reactive In crystalline form, boron is quite unreactive. Elemental boron is a semimetal that is remarkably unreactive. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron forms unique and intricate structures that contain multicenter bonds, in. Reactivity towards acids. Is Boron Stable Or Reactive.
From www.youtube.com
Boron has two stable isotopes, 10B(19) and 11B(81). Calculate average Is Boron Stable Or Reactive Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Reactivity towards acids and alkalis: In crystalline form, boron is quite unreactive. Boron forms unique and intricate structures that contain multicenter bonds, in. Although opaque to visible light, boron can transmit portions of infrared light. Boron is unreactive when comes in contact with acids. Is Boron Stable Or Reactive.
From pubs.acs.org
Insertion of Reactive Rhodium Carbenes into BoronHydrogen Bonds of Is Boron Stable Or Reactive Boron is a poor room temperature. Boron hydrides are used to synthesize organic compounds. It makes stable covalent bonds with other compounds and does not forms. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron forms unique and intricate structures that contain multicenter bonds, in. Boron does not generally make ionic bonds, it forms stable covalent. Is Boron Stable Or Reactive.
From www.toppr.com
Boron has two stable isotopes, ^10B (19) and ^11B(81) . Average Is Boron Stable Or Reactive Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Boron hydrides are used to synthesize organic compounds. Elemental boron is a semimetal that is remarkably unreactive. Reactivity towards acids and alkalis: Amorphous form of boron is reactive. It makes. Is Boron Stable Or Reactive.
From www.slideserve.com
PPT Boron (p102) PowerPoint Presentation, free download ID947998 Is Boron Stable Or Reactive Boron hydrides are used to synthesize organic compounds. Elemental boron is a semimetal that is remarkably unreactive. Boron does not generally make ionic bonds, it forms stable covalent bonds. In crystalline form, boron is quite unreactive. Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Boron is a poor room temperature. Although opaque to visible light, boron. Is Boron Stable Or Reactive.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table Is Boron Stable Or Reactive Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. It makes stable covalent bonds with other compounds and does not forms. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron forms unique and intricate structures that contain multicenter bonds, in. Boron is a poor room temperature. Boron. Is Boron Stable Or Reactive.
From borates.today
Biogeochemical Cycle Of Boron Borates Today Is Boron Stable Or Reactive Boron tends to forms hydrides, the simplest of which is diborane, \(b_2h_6\). Amorphous form of boron is reactive. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. In crystalline form, boron is quite unreactive. Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures. Although opaque to visible light,. Is Boron Stable Or Reactive.
From www.youtube.com
The hybridization of boron in the stable borane having thelowest Is Boron Stable Or Reactive Amorphous form of boron is reactive. Elemental boron is a semimetal that is remarkably unreactive. Although opaque to visible light, boron can transmit portions of infrared light. Boron hydrides are used to synthesize organic compounds. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is a poor room temperature. Boron forms unique. Is Boron Stable Or Reactive.