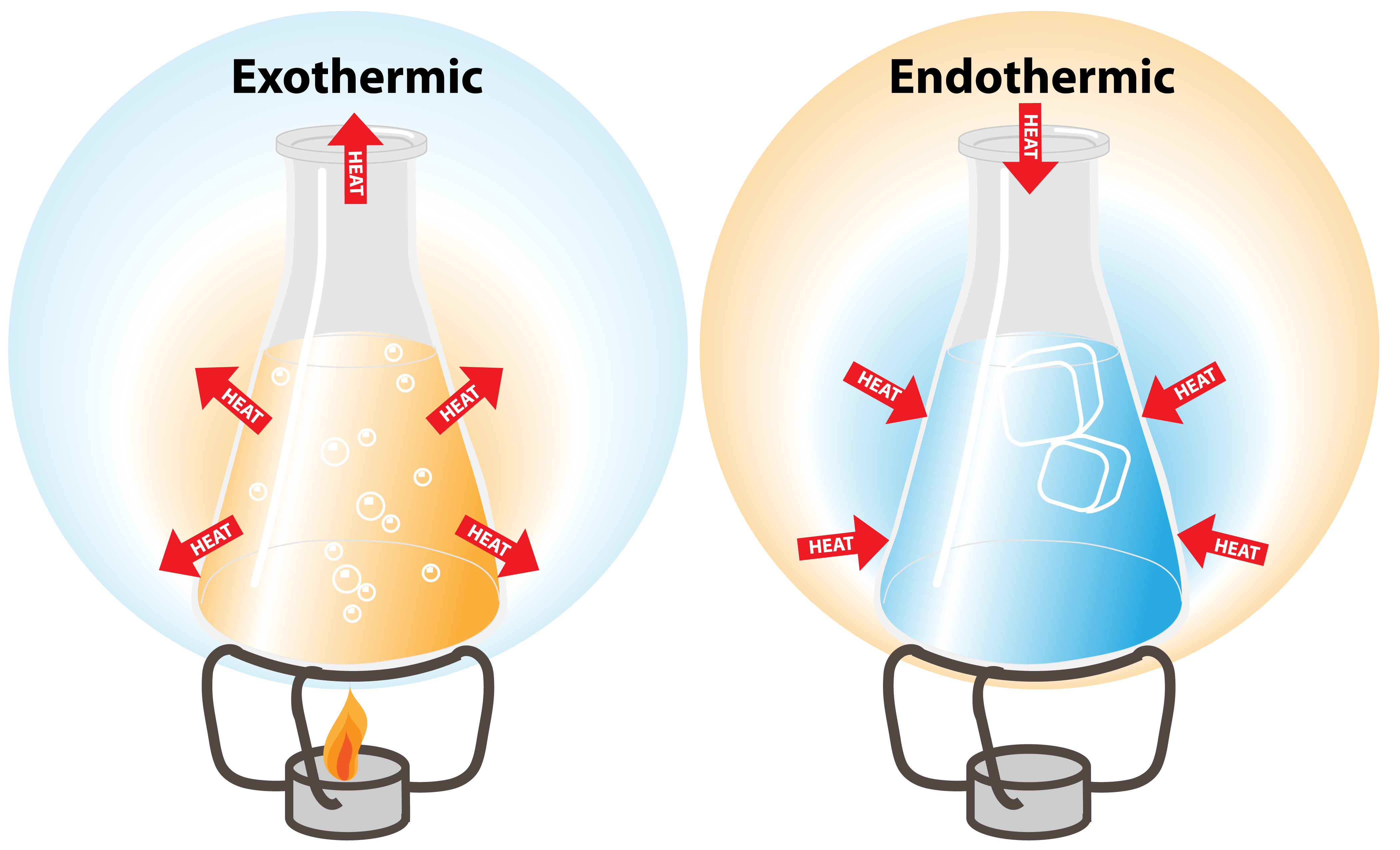
Endothermic Reaction Examples Class 7 . The reaction between ethanoic acid and sodium carbonate. Learn the energy diagram and check out a few examples. Compare and contrast endothermic and exothermic. Some examples of endothermic reactions are: An endothermic reaction is any chemical reaction that absorbs heat from its environment. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Here's a list of examples of endothermic reactions. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Find out about the endothermic reaction. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between.
from www.yaclass.in
An endothermic reaction is any chemical reaction that absorbs heat from its environment. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Some examples of endothermic reactions are: The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Find out about the endothermic reaction. Compare and contrast endothermic and exothermic. Here's a list of examples of endothermic reactions.
Exothermic and Endothermic chemical changes — lesson. Science State
Endothermic Reaction Examples Class 7 The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Here's a list of examples of endothermic reactions. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Learn the energy diagram and check out a few examples. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Some examples of endothermic reactions are: Find out about the endothermic reaction. The reaction between ethanoic acid and sodium carbonate. Compare and contrast endothermic and exothermic. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Examples Class 7 An endothermic reaction is any chemical reaction that absorbs heat from its environment. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Compare and contrast endothermic and exothermic. Learn the energy diagram and check out a few examples. Here's a list of examples of endothermic reactions. The reaction. Endothermic Reaction Examples Class 7.
From www.vrogue.co
Differentiate Between Exothermic And Endothermic Reac vrogue.co Endothermic Reaction Examples Class 7 Here's a list of examples of endothermic reactions. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Compare and contrast endothermic and exothermic. The reaction between ethanoic acid and sodium carbonate. You can use these when asked to cite an example or to get ideas to set up a demonstration of. Endothermic Reaction Examples Class 7.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Class 7 Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is one in which the enthalpy h (or internal energy u). Endothermic Reaction Examples Class 7.
From www.tes.com
Exothermic and Endothermic Reactions Teaching Resources Endothermic Reaction Examples Class 7 Find out about the endothermic reaction. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is any chemical reaction that absorbs heat from. Endothermic Reaction Examples Class 7.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Examples Class 7 An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Revise and understand what endothermic and exothermic reactions are and how. Endothermic Reaction Examples Class 7.
From lessoncampusunspelt.z13.web.core.windows.net
Endothermic And Exothermic Reactions Worksheet Endothermic Reaction Examples Class 7 The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Learn the energy diagram and check out a few examples. Here's a list of examples of endothermic reactions. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Revise and understand. Endothermic Reaction Examples Class 7.
From www.youtube.com
Endothermic Reaction and Exothermic Reaction YouTube Endothermic Reaction Examples Class 7 Compare and contrast endothermic and exothermic. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. The reaction between ethanoic acid and sodium carbonate.. Endothermic Reaction Examples Class 7.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction Examples Class 7 Find out about the endothermic reaction. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Learn the energy diagram and check out a few examples. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Revise and understand what endothermic. Endothermic Reaction Examples Class 7.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Reaction Examples Class 7 The reaction between ethanoic acid and sodium carbonate. Find out about the endothermic reaction. Some examples of endothermic reactions are: You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is one in which the enthalpy h (or internal energy u) of. Endothermic Reaction Examples Class 7.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Examples Class 7 You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Some examples of endothermic reactions are: Learn the energy diagram and check out a few examples. Here's a list of examples of endothermic reactions. The reaction between ethanoic acid and sodium carbonate. Compare and contrast. Endothermic Reaction Examples Class 7.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Examples Class 7 The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Here's a list of examples of endothermic reactions. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is any chemical reaction that absorbs. Endothermic Reaction Examples Class 7.
From scienceinfo.com
Endothermic Reaction with Interesting Examples Endothermic Reaction Examples Class 7 Some examples of endothermic reactions are: You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Learn the energy diagram and check out a few examples. Here's a list of examples of. Endothermic Reaction Examples Class 7.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Examples Class 7 Some examples of endothermic reactions are: Learn the energy diagram and check out a few examples. Find out about the endothermic reaction. Compare and contrast endothermic and exothermic. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and. Endothermic Reaction Examples Class 7.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Examples Class 7 Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Find out about. Endothermic Reaction Examples Class 7.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Class 7 Here's a list of examples of endothermic reactions. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and. Endothermic Reaction Examples Class 7.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Class 7 You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Here's a list of examples of endothermic reactions. Learn the energy diagram and check out a few examples. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system. Endothermic Reaction Examples Class 7.
From studymind.co.uk
Endothermic vs Exothermic Reactions (GCSE Chemistry) Study Mind Endothermic Reaction Examples Class 7 An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction. Endothermic Reaction Examples Class 7.
From mungfali.com
Endothermic Vs Exothermic Reactions Examples Endothermic Reaction Examples Class 7 An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Compare and contrast endothermic and exothermic. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to. Endothermic Reaction Examples Class 7.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Class 7 The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Find out about the endothermic reaction. An endothermic reaction is any chemical reaction that absorbs heat from. Endothermic Reaction Examples Class 7.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Endothermic Reaction Examples Class 7 Find out about the endothermic reaction. Here's a list of examples of endothermic reactions. An endothermic reaction is any chemical reaction that absorbs heat from its environment. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. You can use these when asked to cite an example or to get ideas to. Endothermic Reaction Examples Class 7.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Examples Class 7 Learn the energy diagram and check out a few examples. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Compare and contrast endothermic and exothermic. You can use these when asked to cite an example or to get ideas to set up a. Endothermic Reaction Examples Class 7.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Endothermic Reaction Examples Class 7 Here's a list of examples of endothermic reactions. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is any chemical reaction that absorbs. Endothermic Reaction Examples Class 7.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Class 7 Here's a list of examples of endothermic reactions. An endothermic reaction is any chemical reaction that absorbs heat from its environment. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Compare and contrast endothermic and exothermic. The reaction between ethanoic acid and sodium carbonate.. Endothermic Reaction Examples Class 7.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Class 7 Here's a list of examples of endothermic reactions. The reaction between ethanoic acid and sodium carbonate. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Learn the energy diagram and check out a few examples. You can use these when asked to cite an example or to get ideas to set. Endothermic Reaction Examples Class 7.
From www.pinterest.com
Endothermic Reaction Detailed Explanation with Examples Chemistry Endothermic Reaction Examples Class 7 The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. Compare and contrast endothermic and exothermic. Some examples of endothermic reactions are: The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Find out about the. Endothermic Reaction Examples Class 7.
From www.tes.com
Exothermic and Endothermic Reactions Teaching Resources Endothermic Reaction Examples Class 7 Some examples of endothermic reactions are: Find out about the endothermic reaction. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Here's a list of examples of endothermic reactions. Learn the energy diagram and check out a few examples. The slideshow describes an exothermic reaction between dilute sodium. Endothermic Reaction Examples Class 7.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Examples Class 7 The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The reaction between ethanoic acid and sodium carbonate. Here's a list of examples of endothermic reactions. Learn the energy diagram and. Endothermic Reaction Examples Class 7.
From www.youtube.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Examples Class 7 An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is any chemical reaction that absorbs heat from its environment. The reaction between ethanoic. Endothermic Reaction Examples Class 7.
From www.vrogue.co
Endothermic Reaction Definition Examples Chemical Equ vrogue.co Endothermic Reaction Examples Class 7 Learn the energy diagram and check out a few examples. Some examples of endothermic reactions are: An endothermic reaction is any chemical reaction that absorbs heat from its environment. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Here's a list of examples of endothermic reactions. An endothermic reaction is one. Endothermic Reaction Examples Class 7.
From mavink.com
Endothermic And Exothermic Reactions Examples Endothermic Reaction Examples Class 7 Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Some examples of endothermic reactions are: The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. An endothermic reaction is one in which the enthalpy h (or internal energy u) of. Endothermic Reaction Examples Class 7.
From www.doubtnut.com
Distinguish between Endothermic reaction and Exothermic reactions. Endothermic Reaction Examples Class 7 The reaction between ethanoic acid and sodium carbonate. Learn the energy diagram and check out a few examples. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Compare and contrast endothermic and exothermic. An endothermic reaction is any chemical reaction that absorbs heat from. Endothermic Reaction Examples Class 7.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Examples Class 7 Find out about the endothermic reaction. The reaction between ethanoic acid and sodium carbonate. Here's a list of examples of endothermic reactions. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction is one in which the enthalpy h (or internal energy. Endothermic Reaction Examples Class 7.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Examples Class 7 You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Here's a list of examples of endothermic reactions. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Revise and understand what endothermic and exothermic reactions are and how the two reactions. Endothermic Reaction Examples Class 7.
From ask.modifiyegaraj.com
Endothermic Reaction Examples Science Struck Asking List Endothermic Reaction Examples Class 7 You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. Some examples of endothermic reactions are: Here's a list of examples of endothermic reactions. Compare and contrast endothermic and exothermic. The reaction between ethanoic acid and sodium carbonate. The slideshow describes an exothermic reaction between. Endothermic Reaction Examples Class 7.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Examples Class 7 Compare and contrast endothermic and exothermic. An endothermic reaction is one in which the enthalpy h (or internal energy u) of the system increases. The reaction between ethanoic acid and sodium carbonate. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. An endothermic reaction. Endothermic Reaction Examples Class 7.