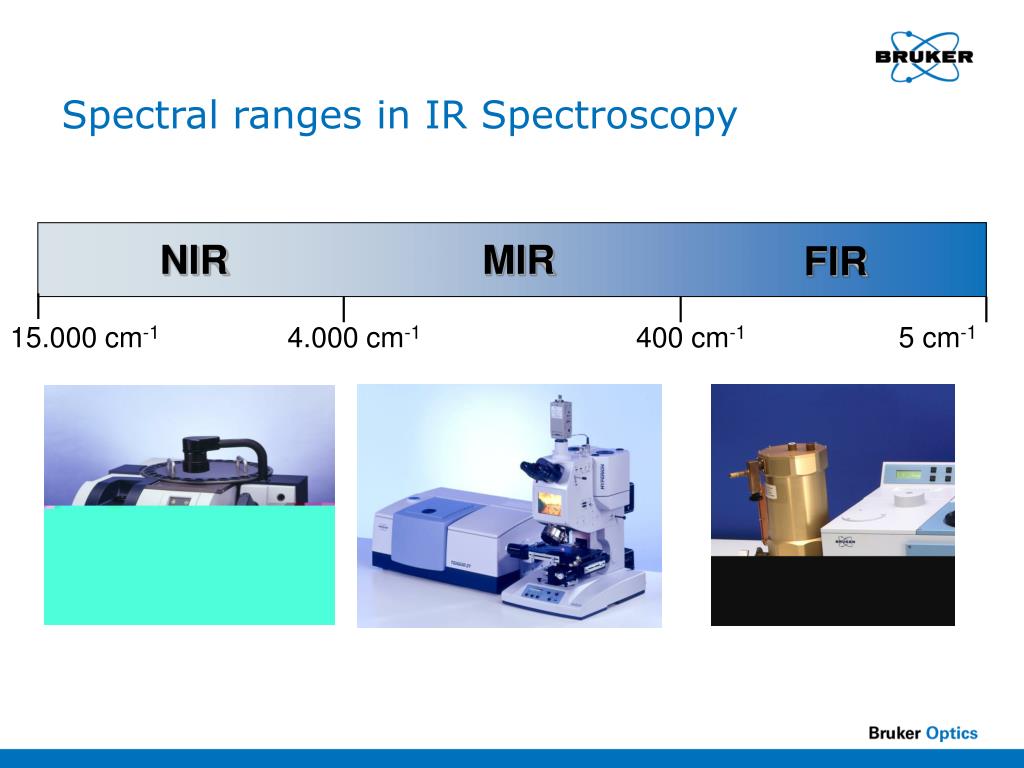
Spectroscopy Range . Emission and photoluminescence spectroscopy use thermal,. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. In one class of techniques there is a transfer of energy. In absorption and scattering spectroscopy this energy is supplied by photons. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. A continuous spectrum shows a. Continuous spectra and discontinuous (or line) spectra. Atomic spectra can be broadly classified into two types based on their appearance: Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. All forms of spectroscopy require a source of energy. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. We will divide spectroscopy into two broad classes of techniques.
from www.slideserve.com
Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. All forms of spectroscopy require a source of energy. Emission and photoluminescence spectroscopy use thermal,. Atomic spectra can be broadly classified into two types based on their appearance: A continuous spectrum shows a. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Continuous spectra and discontinuous (or line) spectra. In absorption and scattering spectroscopy this energy is supplied by photons. In one class of techniques there is a transfer of energy. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy.
PPT Spectral ranges in IR Spectroscopy PowerPoint Presentation, free download ID693807
Spectroscopy Range In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. In absorption and scattering spectroscopy this energy is supplied by photons. A continuous spectrum shows a. Continuous spectra and discontinuous (or line) spectra. We will divide spectroscopy into two broad classes of techniques. In one class of techniques there is a transfer of energy. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. All forms of spectroscopy require a source of energy. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Emission and photoluminescence spectroscopy use thermal,. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. Atomic spectra can be broadly classified into two types based on their appearance:
From www.smorescience.com
Visible Light Spectrum Wavelengths Poster Free Download Smore Science Spectroscopy Range Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In one class of techniques there is a transfer of energy. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. A continuous spectrum shows a. Atomic spectra can be broadly classified into two types based on. Spectroscopy Range.
From www.slideserve.com
PPT Spectral ranges in IR Spectroscopy PowerPoint Presentation, free download ID693807 Spectroscopy Range We will divide spectroscopy into two broad classes of techniques. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In one class of techniques there is a transfer of energy. All forms of spectroscopy require a source of energy. Continuous spectra and. Spectroscopy Range.
From seos-project.eu
Understanding Spectra from the Earth Spectroscopy Range Emission and photoluminescence spectroscopy use thermal,. In absorption and scattering spectroscopy this energy is supplied by photons. We will divide spectroscopy into two broad classes of techniques. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. Continuous spectra and discontinuous (or line) spectra. All forms. Spectroscopy Range.
From kidspressmagazine.com
Visible Light and the Spectrum Spectroscopy Range We will divide spectroscopy into two broad classes of techniques. Atomic spectra can be broadly classified into two types based on their appearance: A continuous spectrum shows a. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Continuous spectra and discontinuous (or line) spectra. Spectroscopy now covers a sizable fraction of. Spectroscopy Range.
From flexautomotive.net
EMC FLEX BLOG Spectrum Spectroscopy Range Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. A continuous spectrum shows a. The table summarizes the. Spectroscopy Range.
From ruby-sapphire.com
Introduction to Infrared Spectroscopy (FTIR) in Gemology Spectroscopy Range Atomic spectra can be broadly classified into two types based on their appearance: All forms of spectroscopy require a source of energy. We will divide spectroscopy into two broad classes of techniques. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. In one class of techniques there is a transfer. Spectroscopy Range.
From lui.ucr.edu
LUI Lab Spectroscopy Range All forms of spectroscopy require a source of energy. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. We will divide spectroscopy into two broad classes of techniques. In one class of techniques there is a transfer of energy. A continuous spectrum shows a. In absorption and scattering spectroscopy this energy is supplied by photons. Spectroscopy. Spectroscopy Range.
From www.dreamstime.com
Light Spectrum stock illustration. Image of design, infographic 68782732 Spectroscopy Range Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. In one class of techniques there is a transfer of energy. Emission and photoluminescence spectroscopy use thermal,. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. We will divide spectroscopy into two broad classes of. Spectroscopy Range.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Spectroscopy Range Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. In one class of techniques there is a transfer of energy. In absorption and scattering spectroscopy this energy is supplied by photons. A continuous spectrum shows. Spectroscopy Range.
From chem.libretexts.org
Raman spectroscopy Chemistry LibreTexts Spectroscopy Range Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Emission and photoluminescence spectroscopy use thermal,. We will divide spectroscopy into two broad classes of techniques. In one class of techniques there is a transfer of energy. Continuous spectra and discontinuous (or line) spectra. Atomic spectra can be broadly classified into two types based on their appearance: Spectroscopy is a. Spectroscopy Range.
From bbpsales.com
Near Infrared Measurements How Do They Work? BBP Spectroscopy Range In one class of techniques there is a transfer of energy. In absorption and scattering spectroscopy this energy is supplied by photons. Continuous spectra and discontinuous (or line) spectra. A continuous spectrum shows a. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. All forms of spectroscopy require a source of energy. We will divide spectroscopy. Spectroscopy Range.
From ar.inspiredpencil.com
Visible Light Spectrum Wavelength Chart Spectroscopy Range In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. In one class of techniques there is a transfer of energy. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. All forms of spectroscopy require a source of energy. Atomic spectra can be broadly classified into two types. Spectroscopy Range.
From armstrongeconomics.com
The Schema Frequency Armstrong Economics Spectroscopy Range In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In one class of techniques there is a transfer of energy. Emission and photoluminescence spectroscopy use thermal,. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Atomic spectra can be broadly classified. Spectroscopy Range.
From leadertechinc.com
Basics of the Spectrum Leader Tech Spectroscopy Range All forms of spectroscopy require a source of energy. In one class of techniques there is a transfer of energy. Atomic spectra can be broadly classified into two types based on their appearance: Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational,. Spectroscopy Range.
From webbtelescope.org
The Spectrum (with Hubble, b, and Spitzer Highlights) b Spectroscopy Range Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In absorption and scattering spectroscopy this energy is supplied by photons. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. In one class of techniques there is a transfer. Spectroscopy Range.
From designroom.com
The World in Infrared designRoom Creative Spectroscopy Range In absorption and scattering spectroscopy this energy is supplied by photons. In one class of techniques there is a transfer of energy. Atomic spectra can be broadly classified into two types based on their appearance: Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. A. Spectroscopy Range.
From www.sciencefacts.net
Visible Light Definition, Wavelength, Uses, and Pictures Spectroscopy Range Emission and photoluminescence spectroscopy use thermal,. In one class of techniques there is a transfer of energy. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. A continuous spectrum shows a. We will divide spectroscopy into two broad classes of techniques. Spectroscopy now covers a. Spectroscopy Range.
From www.infohow.org
Spectrum Spectroscopy Range The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. We will divide spectroscopy into two broad classes of techniques. Emission and photoluminescence spectroscopy use thermal,. Atomic spectra can be broadly classified into two types based on their appearance: All forms of spectroscopy require a source of energy. A continuous spectrum shows a. Continuous spectra and discontinuous. Spectroscopy Range.
From waves.neocities.org
The Spectrum Spectroscopy Range Continuous spectra and discontinuous (or line) spectra. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In absorption and scattering spectroscopy this energy is supplied by photons. Spectroscopy now covers a sizable fraction of the electromagnetic. Spectroscopy Range.
From www.alamy.com
Visible color spectrum. Sunlight wavelength and increasing frequency vector infographic Spectroscopy Range Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. In absorption and scattering spectroscopy this energy is supplied by photons. A continuous spectrum shows a. All forms of spectroscopy require a source of energy. In one class of techniques there is a transfer of energy. Atomic spectra can be broadly classified into two types based on their appearance: Infrared. Spectroscopy Range.
From avsalo.weebly.com
Visible light spectrum avsalo Spectroscopy Range We will divide spectroscopy into two broad classes of techniques. A continuous spectrum shows a. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Continuous spectra and discontinuous (or line) spectra. Emission and photoluminescence spectroscopy use thermal,. Infrared spectroscopy, ultraviolet spectroscopy,. Spectroscopy Range.
From imagine.gsfc.nasa.gov
Spectra Introduction Spectroscopy Range All forms of spectroscopy require a source of energy. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Emission and photoluminescence spectroscopy use thermal,. Continuous spectra and discontinuous (or line) spectra. Atomic spectra can be broadly classified into two types based on their appearance: In one class of techniques there is a transfer of energy. Spectroscopy is a field. Spectroscopy Range.
From www.freepik.com
Premium Vector Spectrum wavelength Visible spectrum color range Educational physics light line Spectroscopy Range In one class of techniques there is a transfer of energy. We will divide spectroscopy into two broad classes of techniques. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Continuous spectra and discontinuous (or line) spectra. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. A continuous spectrum shows a. Spectroscopy is a field of study that investigates. Spectroscopy Range.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Spectroscopy Range Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. We will divide spectroscopy into two broad classes of techniques. Continuous spectra and discontinuous (or line) spectra. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. In one class of techniques there is a transfer of energy. In spectroscopy, we use light to determine a tremendous range of molecular properties,. Spectroscopy Range.
From creativemarket.com
Spectrum wavelength. Visible Education Illustrations Creative Market Spectroscopy Range Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. Emission and photoluminescence spectroscopy use thermal,. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. In absorption and scattering spectroscopy this energy is supplied by photons. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. All forms of. Spectroscopy Range.
From www.thoughtco.com
Visible Light Spectrum Overview and Chart Spectroscopy Range All forms of spectroscopy require a source of energy. A continuous spectrum shows a. Continuous spectra and discontinuous (or line) spectra. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. Emission and photoluminescence spectroscopy use thermal,. Infrared spectroscopy,. Spectroscopy Range.
From courses.lumenlearning.com
Spectroscopy in Astronomy Astronomy Spectroscopy Range The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. All forms of spectroscopy require a source of energy. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. We will divide spectroscopy into two broad classes of techniques. Atomic spectra can be broadly classified into two types based on their appearance: In spectroscopy, we use light to. Spectroscopy Range.
From chem.libretexts.org
10 Introduction to Spectroscopy Chemistry LibreTexts Spectroscopy Range Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. Continuous spectra and discontinuous (or line) spectra. The table summarizes the electromagnetic spectrum over a frequency range of 16 orders. All forms of spectroscopy require a source of energy. Atomic spectra can be broadly classified into two types based on their appearance: In spectroscopy, we use light to determine a tremendous. Spectroscopy Range.
From imagine.gsfc.nasa.gov
Spectrum Spectroscopy Range Continuous spectra and discontinuous (or line) spectra. In absorption and scattering spectroscopy this energy is supplied by photons. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Emission and photoluminescence spectroscopy use thermal,. In one class of techniques there is a transfer of energy. All forms of spectroscopy require a source of energy. Atomic spectra can be broadly classified. Spectroscopy Range.
From hubblesite.org
The Spectrum HubbleSite Spectroscopy Range In one class of techniques there is a transfer of energy. Emission and photoluminescence spectroscopy use thermal,. We will divide spectroscopy into two broad classes of techniques. All forms of spectroscopy require a source of energy. In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Atomic spectra can be broadly classified. Spectroscopy Range.
From www.slideserve.com
PPT Infrared Spectroscopy Theory and Interpretation of IR spectra PowerPoint Presentation ID Spectroscopy Range Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. A continuous spectrum shows a. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. In absorption and scattering spectroscopy this energy is supplied by photons. Continuous. Spectroscopy Range.
From awesomehome.co
Ir Spectra Table Functional Groups Awesome Home Spectroscopy Range In absorption and scattering spectroscopy this energy is supplied by photons. A continuous spectrum shows a. Emission and photoluminescence spectroscopy use thermal,. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. The table summarizes the. Spectroscopy Range.
From cabinet.matttroy.net
Ir Spectrum Table By Frequency Range Matttroy Spectroscopy Range Continuous spectra and discontinuous (or line) spectra. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. Emission and photoluminescence spectroscopy use thermal,. We will divide spectroscopy into two broad classes of techniques. In absorption and scattering. Spectroscopy Range.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Spectroscopy Range In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. In absorption and scattering spectroscopy this energy is supplied by photons. Emission and photoluminescence spectroscopy use thermal,. Atomic spectra can be broadly classified into two types based on their appearance: In one class of techniques there is a transfer of energy. Continuous. Spectroscopy Range.
From www.livescience.com
What Is XRay Spectroscopy? Live Science Spectroscopy Range In spectroscopy, we use light to determine a tremendous range of molecular properties, including electronic, vibrational, rotational, and. Continuous spectra and discontinuous (or line) spectra. Spectroscopy now covers a sizable fraction of the electromagnetic spectrum. Infrared spectroscopy, ultraviolet spectroscopy, and nuclear magnetic resonance spectroscopy. All forms of spectroscopy require a source of energy. Spectroscopy is a field of study that. Spectroscopy Range.