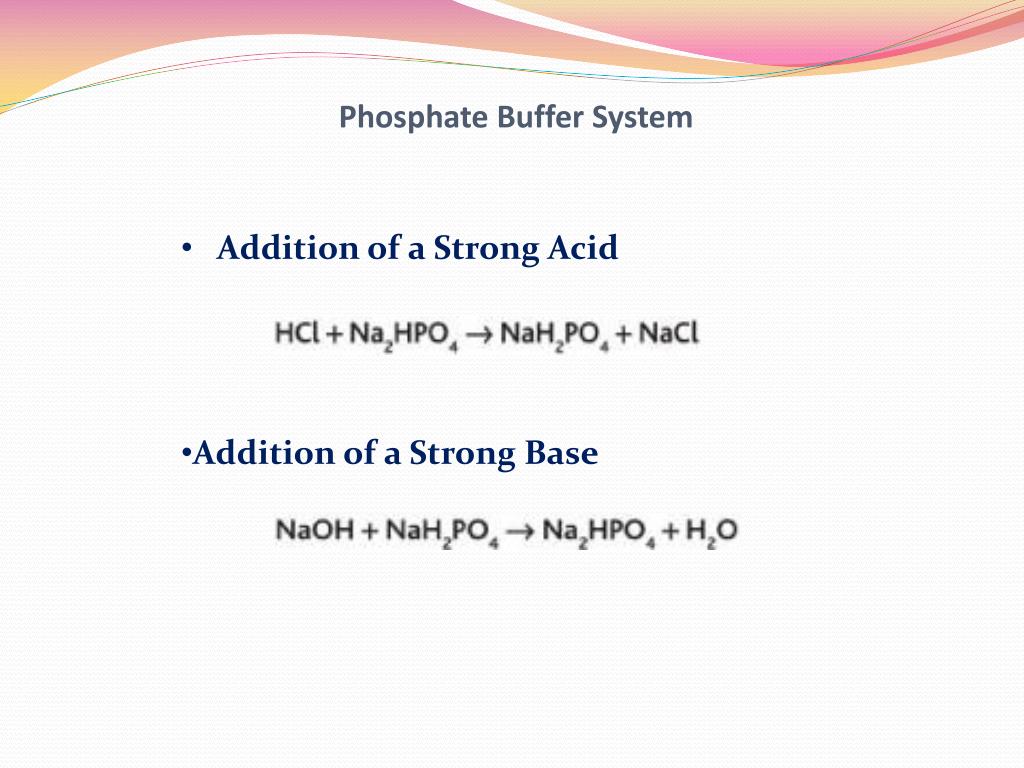
Buffer In Body Fluids . a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. the body uses buffer systems to reduce the impact of an acute acid load. Bicarbonate is the most important buffer. buffers are solutions that contain a weak acid and its a conjugate base; various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. other buffer systems in the human body include the phosphate.
from www.slideserve.com
other buffer systems in the human body include the phosphate. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. buffers are solutions that contain a weak acid and its a conjugate base; a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. the body uses buffer systems to reduce the impact of an acute acid load. Bicarbonate is the most important buffer. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them.
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation
Buffer In Body Fluids other buffer systems in the human body include the phosphate. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. other buffer systems in the human body include the phosphate. Bicarbonate is the most important buffer. the body uses buffer systems to reduce the impact of an acute acid load. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. buffers are solutions that contain a weak acid and its a conjugate base;
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2821926 Buffer In Body Fluids buffers are solutions that contain a weak acid and its a conjugate base; various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. Bicarbonate is the most important buffer. the body uses buffer systems to reduce the impact of an acute acid load.. Buffer In Body Fluids.
From www.pinterest.com
Buffers in the body Fluid and electrolytes, Extracellular fluid, Acid Buffer In Body Fluids buffers are solutions that contain a weak acid and its a conjugate base; various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. other buffer systems in the human body include the phosphate. the body uses buffer systems to reduce the impact. Buffer In Body Fluids.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Buffer In Body Fluids a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. buffers are solutions that contain a weak acid and its a conjugate base; Bicarbonate is the most important buffer. other buffer systems in the human body include the phosphate. various buffer systems exist in body fluids. Buffer In Body Fluids.
From www.slideserve.com
PPT 1. Body Fluid Compartments PowerPoint Presentation, free download Buffer In Body Fluids a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. other buffer systems in the human body include the phosphate. buffers are solutions that contain a weak acid and its a conjugate base; the body uses buffer systems to reduce the impact of an acute acid. Buffer In Body Fluids.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Buffer In Body Fluids a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. the body uses buffer systems to reduce the impact of an acute acid load. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. . Buffer In Body Fluids.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 Buffer In Body Fluids various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. buffers are solutions that contain a weak acid and its a conjugate base; other buffer systems in the human body include the phosphate. Bicarbonate is the most important buffer. the body uses. Buffer In Body Fluids.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Buffer In Body Fluids Bicarbonate is the most important buffer. the body uses buffer systems to reduce the impact of an acute acid load. buffers are solutions that contain a weak acid and its a conjugate base; a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. various buffer systems. Buffer In Body Fluids.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Buffer In Body Fluids a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. Bicarbonate is the most important buffer. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. buffers are solutions that contain a weak acid and. Buffer In Body Fluids.
From present5.com
Fundamentals of General Organic and Biological Chemistry 6 Buffer In Body Fluids the body uses buffer systems to reduce the impact of an acute acid load. other buffer systems in the human body include the phosphate. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. various buffer systems exist in body fluids (see table) to minimise the. Buffer In Body Fluids.
From www.slideserve.com
PPT (Renal Physiology 10) AcidBase Balance 2 Buffers System Buffer In Body Fluids a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. the body uses buffer systems to reduce the impact of an acute acid load. Bicarbonate is the most important buffer. a buffer is a substance that prevents a radical change in fluid ph by absorbing. Buffer In Body Fluids.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Buffer In Body Fluids a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. Bicarbonate is the most important buffer. buffers are solutions that contain a weak acid and its a conjugate base; a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change. Buffer In Body Fluids.
From slideplayer.com
Introduction to Biology ppt download Buffer In Body Fluids a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. the body uses buffer systems to reduce the impact of an acute acid load. other buffer systems in the human body include the phosphate. various buffer systems exist in body fluids (see table) to. Buffer In Body Fluids.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffer In Body Fluids Bicarbonate is the most important buffer. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. the body uses buffer systems to reduce the impact of an acute acid load. other buffer systems in the human body include the phosphate. buffers are solutions that contain a. Buffer In Body Fluids.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5608285 Buffer In Body Fluids Bicarbonate is the most important buffer. the body uses buffer systems to reduce the impact of an acute acid load. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the. Buffer In Body Fluids.
From www.slideshare.net
Acid base balance Buffer In Body Fluids Bicarbonate is the most important buffer. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. other buffer systems in the human body include the phosphate. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen. Buffer In Body Fluids.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Buffer In Body Fluids Bicarbonate is the most important buffer. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. buffers are solutions that contain. Buffer In Body Fluids.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Buffer In Body Fluids the body uses buffer systems to reduce the impact of an acute acid load. buffers are solutions that contain a weak acid and its a conjugate base; other buffer systems in the human body include the phosphate. Bicarbonate is the most important buffer. a buffer is a chemical system that prevents a radical change in fluid. Buffer In Body Fluids.
From www.chegg.com
Solved An important buffer in body fluids isNaHCO 3NaOHH 2 Buffer In Body Fluids a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. the body uses buffer systems to reduce the impact of an acute acid load. other buffer systems in the human body include the phosphate. Bicarbonate is the most important buffer. buffers are solutions that. Buffer In Body Fluids.
From www.ehow.com
What Are the Three Buffer Systems in Body Fluid? Healthfully Buffer In Body Fluids other buffer systems in the human body include the phosphate. Bicarbonate is the most important buffer. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of. Buffer In Body Fluids.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 Buffer In Body Fluids various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. Bicarbonate is the most important buffer. the body uses buffer systems to reduce the impact of an acute acid load. a buffer is a substance that prevents a radical change in fluid ph. Buffer In Body Fluids.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Buffer In Body Fluids other buffer systems in the human body include the phosphate. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. . Buffer In Body Fluids.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2821926 Buffer In Body Fluids Bicarbonate is the most important buffer. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. other buffer systems in the human body include the phosphate. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen. Buffer In Body Fluids.
From www.slideshare.net
Buffer system Buffer In Body Fluids other buffer systems in the human body include the phosphate. the body uses buffer systems to reduce the impact of an acute acid load. buffers are solutions that contain a weak acid and its a conjugate base; Bicarbonate is the most important buffer. a buffer is a substance that prevents a radical change in fluid ph. Buffer In Body Fluids.
From www.youtube.com
Acid base balance PART 1 BUFFERS IN BODY FLUIDS & HENDERSON HASSELBACH Buffer In Body Fluids a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. Bicarbonate is the most important buffer. various buffer systems exist in body fluids (see table). Buffer In Body Fluids.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Buffer In Body Fluids buffers are solutions that contain a weak acid and its a conjugate base; the body uses buffer systems to reduce the impact of an acute acid load. Bicarbonate is the most important buffer. other buffer systems in the human body include the phosphate. a buffer is a substance that prevents a radical change in fluid ph. Buffer In Body Fluids.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body Buffer In Body Fluids buffers are solutions that contain a weak acid and its a conjugate base; a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. Bicarbonate is the most important buffer. other buffer systems in the human body include the phosphate. a buffer is a chemical system that. Buffer In Body Fluids.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Buffer In Body Fluids a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. the body uses buffer systems to reduce the impact of an acute acid load. Bicarbonate is the most important buffer. various buffer systems exist in body fluids (see table) to minimise the effects on ph. Buffer In Body Fluids.
From www.pinterest.com
chemical and physiological pH buffers Biochemistry notes, Nursing Buffer In Body Fluids Bicarbonate is the most important buffer. buffers are solutions that contain a weak acid and its a conjugate base; the body uses buffer systems to reduce the impact of an acute acid load. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. various. Buffer In Body Fluids.
From www.slideserve.com
PPT Buffer systems PowerPoint Presentation, free download ID9427698 Buffer In Body Fluids the body uses buffer systems to reduce the impact of an acute acid load. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. . Buffer In Body Fluids.
From www.slideserve.com
PPT Fluid and Electrolyte Balance PowerPoint Presentation, free Buffer In Body Fluids a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. the body uses buffer systems to reduce the impact of an acute acid. Buffer In Body Fluids.
From www.slideserve.com
PPT Buffer systems PowerPoint Presentation, free download ID9427698 Buffer In Body Fluids Bicarbonate is the most important buffer. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. the body uses buffer systems. Buffer In Body Fluids.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Buffer In Body Fluids buffers are solutions that contain a weak acid and its a conjugate base; the body uses buffer systems to reduce the impact of an acute acid load. a buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations. other buffer systems in the human body. Buffer In Body Fluids.
From www.online-sciences.com
Body fluid exchange, The electrical characteristic of the cells Buffer In Body Fluids various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. buffers are solutions that contain a weak acid and its a conjugate base;. Buffer In Body Fluids.
From www.slideserve.com
PPT Fluid and Electrolyte Balance PowerPoint Presentation, free Buffer In Body Fluids the body uses buffer systems to reduce the impact of an acute acid load. buffers are solutions that contain a weak acid and its a conjugate base; Bicarbonate is the most important buffer. various buffer systems exist in body fluids (see table) to minimise the effects on ph of the addition or removal of acid from them.. Buffer In Body Fluids.
From www.scribd.com
pH and Buffer system in Body fluids PDF Lymph Buffer Solution Buffer In Body Fluids buffers are solutions that contain a weak acid and its a conjugate base; other buffer systems in the human body include the phosphate. a buffer is a substance that prevents a radical change in fluid ph by absorbing excess hydrogen or hydroxyl ions. the body uses buffer systems to reduce the impact of an acute acid. Buffer In Body Fluids.