Which Is More Polar Acetone Or Ethanol . 23 rows solvents used in organic chemistry are characterized by their physical characteristics. It is a colorless liquid with a distinct sweet odor. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Among the most important are whether the. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Ethanol, on the other hand, is. Common solvents arranged from the least polar to the most polar Acetone, also known as propanone, has the chemical formula c 3 h 6 o. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. 5 to 30% is more suitable for strongly polar compounds.
from www.numerade.com
In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. 5 to 30% is more suitable for strongly polar compounds. It is a colorless liquid with a distinct sweet odor. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Ethanol is the more polar solvent compared to acetone. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Among the most important are whether the. Common solvents arranged from the least polar to the most polar
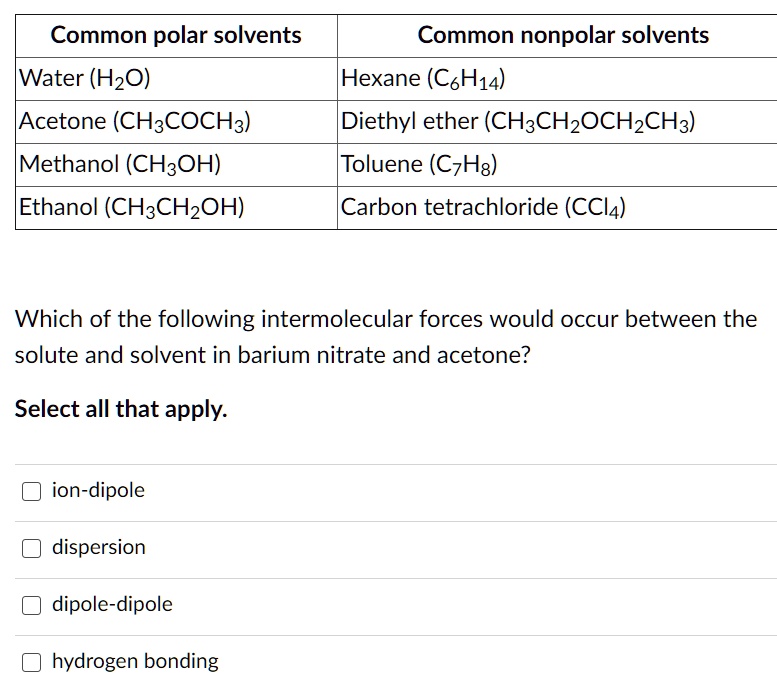
SOLVED Common polar solvents Common nonpolar solvents Water (HzO
Which Is More Polar Acetone Or Ethanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Ethanol, on the other hand, is. 5 to 30% is more suitable for strongly polar compounds. Common solvents arranged from the least polar to the most polar Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Among the most important are whether the. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Ethanol is the more polar solvent compared to acetone. It is a colorless liquid with a distinct sweet odor.
From magdalenakruwsalazar.blogspot.com
Acetone Polar or Nonpolar Solvent MagdalenakruwSalazar Which Is More Polar Acetone Or Ethanol In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Common solvents arranged from the least polar to the most polar It is a colorless liquid with. Which Is More Polar Acetone Or Ethanol.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Which Is More Polar Acetone Or Ethanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Ethanol, on the other hand, is. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. 5 to 30% is more suitable for strongly polar compounds. Among the most important are whether. Which Is More Polar Acetone Or Ethanol.
From byjus.com
34. Acetone is treated with excess of ethanol in the presence of Which Is More Polar Acetone Or Ethanol Ethanol, on the other hand, is. Ethanol is the more polar solvent compared to acetone. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. 23 rows solvents used in organic chemistry. Which Is More Polar Acetone Or Ethanol.
From mungfali.com
Acetone Polar Or Nonpolar Which Is More Polar Acetone Or Ethanol 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Among the most important are whether the. Ethanol is the more polar solvent compared to acetone. Common solvents arranged from the least. Which Is More Polar Acetone Or Ethanol.
From www.chegg.com
Solved Which of these solvents are polar protic? Choose all Which Is More Polar Acetone Or Ethanol 5 to 30% is more suitable for strongly polar compounds. Among the most important are whether the. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Common solvents arranged from the least polar to the most polar In your case, acetone has. Which Is More Polar Acetone Or Ethanol.
From www.youtube.com
a mixture of ethanol and acetone shows positive deviation YouTube Which Is More Polar Acetone Or Ethanol 5 to 30% is more suitable for strongly polar compounds. It is a colorless liquid with a distinct sweet odor. Ethanol, on the other hand, is. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. In your case, acetone has a higher dipole moment. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
Solvent Solvent Polarity Index, P Hexane 0.1 Carbon tetrachloride Which Is More Polar Acetone Or Ethanol Among the most important are whether the. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. 5 to 30% is more suitable for strongly polar compounds. In your case, acetone has a higher dipole moment than ethanol because it has. Which Is More Polar Acetone Or Ethanol.
From courses.lumenlearning.com
Properties of Liquids Chemistry Atoms First Which Is More Polar Acetone Or Ethanol Among the most important are whether the. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Ethanol, on the other hand, is. 5 to 30% is more suitable for strongly polar compounds. Acetone, also known as. Which Is More Polar Acetone Or Ethanol.
From www.researchgate.net
Why we use ACETONE in "acetone insolubility" study instead of other Which Is More Polar Acetone Or Ethanol In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Among the most important are whether the. 5 to 30% is more suitable. Which Is More Polar Acetone Or Ethanol.
From mungfali.com
Acetone Polar Or Nonpolar Which Is More Polar Acetone Or Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Ethanol, on the other hand, is. Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Ethanol is the more polar solvent compared to acetone. Among the most important are whether the. 5. Which Is More Polar Acetone Or Ethanol.
From www.youtube.com
Which Bond is the Most Polar CH, OH, NH, or CC YouTube Which Is More Polar Acetone Or Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. 5 to 30% is more suitable for strongly polar compounds. It is a colorless liquid with a distinct sweet odor. Common solvents arranged from the least polar to the most polar Ethanol is the. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
SOLVED Common polar solvents Common nonpolar solvents Water (HzO Which Is More Polar Acetone Or Ethanol Among the most important are whether the. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Ethanol, on the other hand, is. 5 to 30% is more suitable for strongly polar compounds. It is a colorless liquid with a distinct sweet odor. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
SOLVED Rank hexane, water, ethanol, acetone, and ethyl acetate from Which Is More Polar Acetone Or Ethanol Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Among the most important are whether the. Common solvents arranged from the least polar to the most polar Ethanol, on the other. Which Is More Polar Acetone Or Ethanol.
From exoxnpydf.blob.core.windows.net
Why Is Acetone More Polar Than Ethyl Acetate at Linda Haley blog Which Is More Polar Acetone Or Ethanol Acetone, also known as propanone, has the chemical formula c 3 h 6 o. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Ethanol is the more polar solvent compared. Which Is More Polar Acetone Or Ethanol.
From www.researchgate.net
Polarizability spectra for water, acetone, and ethanol. Each of the Which Is More Polar Acetone Or Ethanol Acetone, also known as propanone, has the chemical formula c 3 h 6 o. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Ethanol, on the other hand, is. 5 to 30% is more suitable for strongly polar compounds. It is a colorless liquid with a. Which Is More Polar Acetone Or Ethanol.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free Which Is More Polar Acetone Or Ethanol It is a colorless liquid with a distinct sweet odor. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Common solvents arranged from the least polar to the most polar Ethanol, on the other hand, is. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Among the most important. Which Is More Polar Acetone Or Ethanol.
From socratic.org
Is methanol a polar molecule? Socratic Which Is More Polar Acetone Or Ethanol It is a colorless liquid with a distinct sweet odor. Ethanol is the more polar solvent compared to acetone. Among the most important are whether the. Ethanol, on the other hand, is. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl.. Which Is More Polar Acetone Or Ethanol.
From www.chemicalbook.com
Is acetonitrile a highly polar solvent?_Chemicalbook Which Is More Polar Acetone Or Ethanol Among the most important are whether the. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Ethanol is the more polar solvent compared to acetone. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. It is a colorless liquid with a distinct sweet odor. Common solvents arranged from the least polar to the. Which Is More Polar Acetone Or Ethanol.
From www.youtube.com
Is C2H5OH Polar or Nonpolar? (Ethanol) YouTube Which Is More Polar Acetone Or Ethanol Ethanol is the more polar solvent compared to acetone. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and. Which Is More Polar Acetone Or Ethanol.
From www.vedantu.com
The most reactive of the following is A Acetone B Benzophenone class 11 Which Is More Polar Acetone Or Ethanol 5 to 30% is more suitable for strongly polar compounds. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Acetone, also known as propanone, has the chemical formula c 3 h 6 o.. Which Is More Polar Acetone Or Ethanol.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? Which Is More Polar Acetone Or Ethanol Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Ethanol is the more polar solvent compared to acetone. Ethanol, on the other hand, is. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine. Which Is More Polar Acetone Or Ethanol.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Which Is More Polar Acetone Or Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. It is a colorless liquid with a distinct sweet odor. Ethanol, on the other hand, is. 5 to 30% is more suitable for strongly polar compounds. Ethanol is the more polar solvent compared to acetone. Among the most important are whether the. 23 rows solvents. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
SOLVED pulal Draw the structures of ethanol, acetone, toluene, hexane Which Is More Polar Acetone Or Ethanol Ethanol, on the other hand, is. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Water acetic acid ethylene. Which Is More Polar Acetone Or Ethanol.
From slideplayer.com
INTERMOLECULAR FORCES OF ATTRACTION ppt download Which Is More Polar Acetone Or Ethanol Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Ethanol is the more polar solvent compared to acetone. Ethanol, on the other hand, is. Common solvents arranged from the least polar to the most polar 5 to 30% is more suitable for strongly polar compounds. It is a colorless liquid with a distinct sweet odor.. Which Is More Polar Acetone Or Ethanol.
From marisol-jolpblogduncan.blogspot.com
Acetone Polar or Nonpolar Solvent Which Is More Polar Acetone Or Ethanol Among the most important are whether the. Ethanol is the more polar solvent compared to acetone. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. 5 to 30% is more suitable for strongly polar compounds. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. In your case, acetone has a. Which Is More Polar Acetone Or Ethanol.
From www.youtube.com
Is C3H6O Polar or NonPolar? (Acetone) YouTube Which Is More Polar Acetone Or Ethanol It is a colorless liquid with a distinct sweet odor. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. 5 to 30% is more suitable for strongly polar compounds. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the. Which Is More Polar Acetone Or Ethanol.
From www.chegg.com
Solved Rank The Following Solvents In Order Of Polarity Which Is More Polar Acetone Or Ethanol It is a colorless liquid with a distinct sweet odor. Among the most important are whether the. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Ethanol, on the other hand, is. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and. Which Is More Polar Acetone Or Ethanol.
From answerlibaccuses.z21.web.core.windows.net
Acetone Is Polar Protic Or Aprotic Which Is More Polar Acetone Or Ethanol Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Ethanol is the more polar solvent compared to acetone. It is a colorless liquid with a distinct sweet odor. Ethanol, on the other hand, is. 5 to 30% is more suitable for strongly polar compounds. Among the most important are whether the. 23 rows solvents used. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
SOLVED Common polar solvents Common nonpolar solvents Water (HzO Which Is More Polar Acetone Or Ethanol It is a colorless liquid with a distinct sweet odor. Common solvents arranged from the least polar to the most polar 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. In your case, acetone has a higher dipole moment than ethanol because. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
SOLVED What is more polar, acetone or hexanes? Which Is More Polar Acetone Or Ethanol 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Ethanol, on the other hand, is. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. 5 to 30% is more suitable for strongly polar compounds. Common solvents arranged from the least. Which Is More Polar Acetone Or Ethanol.
From www.researchgate.net
Possible reaction route between acetone and ethanol (Onyestyák et Which Is More Polar Acetone Or Ethanol Ethanol, on the other hand, is. Ethanol is the more polar solvent compared to acetone. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. It is a colorless liquid with a distinct sweet odor. Common solvents arranged from the least polar. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
Ethanol Dimethyl ether 7th attempt Part 1 (1 point) Using the models Which Is More Polar Acetone Or Ethanol Among the most important are whether the. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. It is a colorless liquid with a distinct sweet odor. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Ethanol,. Which Is More Polar Acetone Or Ethanol.
From www.numerade.com
SOLVED Draw the Lewis structure of acetone, CH3COCH3, and chloroform Which Is More Polar Acetone Or Ethanol 23 rows solvents used in organic chemistry are characterized by their physical characteristics. 5 to 30% is more suitable for strongly polar compounds. Ethanol is the more polar solvent compared to acetone. In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. Among the most important are. Which Is More Polar Acetone Or Ethanol.
From www.youtube.com
Ethanol to acetone YouTube Which Is More Polar Acetone Or Ethanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Among the most important are whether the. Ethanol, on the other hand, is. It is a colorless liquid with a distinct sweet odor. Acetone, also known as propanone, has the chemical formula c 3 h 6 o. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine. Which Is More Polar Acetone Or Ethanol.
From it.wikipedia.org
Acetone Wikipedia Which Is More Polar Acetone Or Ethanol In your case, acetone has a higher dipole moment than ethanol because it has a carbonyl group more towards the center and thus. 23 rows solvents used in organic chemistry are characterized by their physical characteristics. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl. Common solvents arranged from the least polar to the. Which Is More Polar Acetone Or Ethanol.