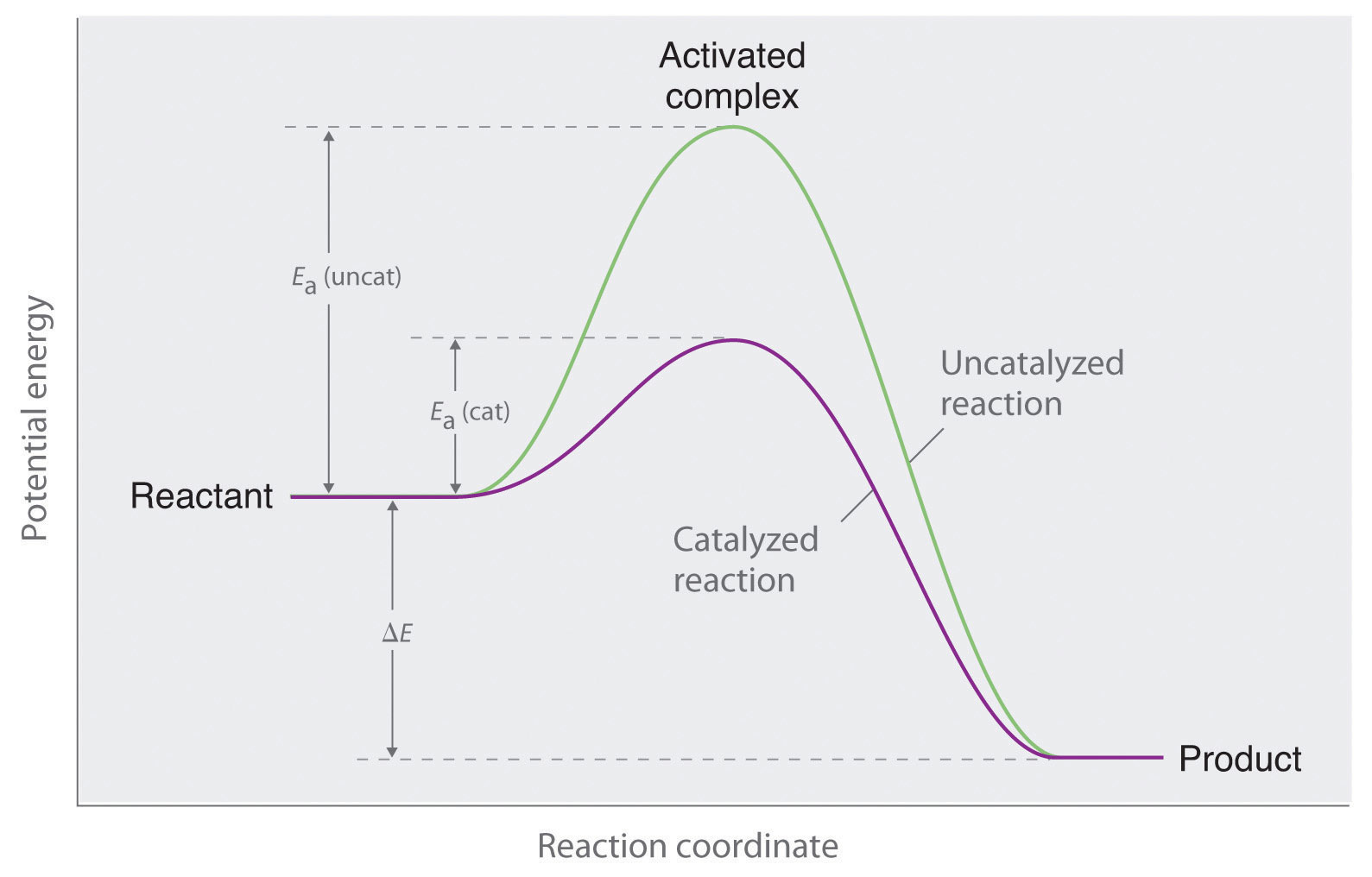
Enzymes Are Examples Of Heterogeneous Catalyst . an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. homogeneous and heterogeneous catalysis: mechanism of an enzyme catalyst. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. a heterogeneous catalyst is present in a different phase from the reactants. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. Catalysis is a phenomenon in which the rate of the reaction is altered. As shown in part (a) in. Such catalysts are usually solids, and often function by furnishing an active. Bridging the gap through surface organometallic chemistry.
from 2012books.lardbucket.org
As shown in part (a) in. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. homogeneous and heterogeneous catalysis: Bridging the gap through surface organometallic chemistry. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Such catalysts are usually solids, and often function by furnishing an active. mechanism of an enzyme catalyst. a heterogeneous catalyst is present in a different phase from the reactants.
Catalysis
Enzymes Are Examples Of Heterogeneous Catalyst As shown in part (a) in. Catalysis is a phenomenon in which the rate of the reaction is altered. As shown in part (a) in. mechanism of an enzyme catalyst. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. a heterogeneous catalyst is present in a different phase from the reactants. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Such catalysts are usually solids, and often function by furnishing an active. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. Bridging the gap through surface organometallic chemistry. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. homogeneous and heterogeneous catalysis:
From www.researchgate.net
Mechanism of a general base heterogeneous catalyst during... Download Enzymes Are Examples Of Heterogeneous Catalyst Bridging the gap through surface organometallic chemistry. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. As shown in part (a) in. a heterogeneous. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Heterogeneous Catalysis & Solid State Physics PowerPoint Enzymes Are Examples Of Heterogeneous Catalyst A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. As shown in part (a) in. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Heterogeneous catalysts are catalysts that speed up the rate of. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideshare.net
Catalysis Enzymes Are Examples Of Heterogeneous Catalyst A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. a heterogeneous catalyst is present in a different phase from the reactants. Catalysis is a phenomenon in which the rate of the reaction is altered. homogeneous and heterogeneous catalysis: Such catalysts are usually solids, and often function by furnishing an. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID226444 Enzymes Are Examples Of Heterogeneous Catalyst homogeneous and heterogeneous catalysis: mechanism of an enzyme catalyst. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Such catalysts are usually solids, and often. Enzymes Are Examples Of Heterogeneous Catalyst.
From alchetron.com
Heterogeneous catalysis Alchetron, the free social encyclopedia Enzymes Are Examples Of Heterogeneous Catalyst homogeneous and heterogeneous catalysis: an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. an example of heterogeneous catalysis is using a solid catalyst. Enzymes Are Examples Of Heterogeneous Catalyst.
From pubs.acs.org
Heterogeneous Catalysis A Central Science for a Sustainable Future Enzymes Are Examples Of Heterogeneous Catalyst As shown in part (a) in. mechanism of an enzyme catalyst. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Bridging the gap through surface organometallic chemistry. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.numerade.com
SOLVED Are enzymecatalyzed reactions examples of homogeneous or Enzymes Are Examples Of Heterogeneous Catalyst a heterogeneous catalyst is present in a different phase from the reactants. Bridging the gap through surface organometallic chemistry. mechanism of an enzyme catalyst. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. As shown in part (a) in. an example of heterogeneous catalysis is using. Enzymes Are Examples Of Heterogeneous Catalyst.
From slideplayer.com
Catalyst Catalysis. ppt download Enzymes Are Examples Of Heterogeneous Catalyst a heterogeneous catalyst is present in a different phase from the reactants. mechanism of an enzyme catalyst. Bridging the gap through surface organometallic chemistry. homogeneous and heterogeneous catalysis: Such catalysts are usually solids, and often function by furnishing an active. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Enzymes Are Examples Of Heterogeneous Catalyst an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. As shown in part (a) in. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. A heterogeneous catalyst is a catalyst that is. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.researchgate.net
Use of heterogeneous catalysis in various chemistry sectors Download Enzymes Are Examples Of Heterogeneous Catalyst homogeneous and heterogeneous catalysis: a heterogeneous catalyst is present in a different phase from the reactants. Bridging the gap through surface organometallic chemistry. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. As shown in part (a) in. Heterogeneous catalysts are. Enzymes Are Examples Of Heterogeneous Catalyst.
From chem.libretexts.org
17.6 Catalysts and Catalysis Chemistry LibreTexts Enzymes Are Examples Of Heterogeneous Catalyst a heterogeneous catalyst is present in a different phase from the reactants. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. mechanism of an enzyme catalyst. As shown in part (a) in. an example of heterogeneous catalysis is the interaction. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Organometallic Reactions and Catalysis PowerPoint Presentation Enzymes Are Examples Of Heterogeneous Catalyst Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. Catalysis is a phenomenon in which the rate of the reaction is altered. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. mechanism of. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.researchgate.net
Homogeneous vs heterogeneous catalysts. Design of heterogeneous Enzymes Are Examples Of Heterogeneous Catalyst A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. As shown in part (a) in. mechanism of an enzyme catalyst. Bridging the gap through surface organometallic chemistry. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Enzymes Are Examples Of Heterogeneous Catalyst an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. a heterogeneous catalyst is present in a different phase from the reactants. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. an example of heterogeneous. Enzymes Are Examples Of Heterogeneous Catalyst.
From en.ppt-online.org
Heterogeneous catalysis online presentation Enzymes Are Examples Of Heterogeneous Catalyst a heterogeneous catalyst is present in a different phase from the reactants. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Bridging the gap through surface organometallic chemistry. homogeneous and heterogeneous catalysis: Catalysis is a phenomenon in which the rate of. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.sliderbase.com
Catalytic Activity Enzymes Are Examples Of Heterogeneous Catalyst an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. Bridging the gap through surface organometallic chemistry. a heterogeneous catalyst is present in a different phase from the reactants. mechanism of an enzyme catalyst. homogeneous and heterogeneous catalysis: A heterogeneous catalyst is a. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Heterogeneous catalysis PowerPoint Presentation, free download Enzymes Are Examples Of Heterogeneous Catalyst Such catalysts are usually solids, and often function by furnishing an active. mechanism of an enzyme catalyst. As shown in part (a) in. Bridging the gap through surface organometallic chemistry. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. A heterogeneous catalyst is a. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.pinterest.com
Heterogeneous Catalyst Easy Science 10th grade science, Ap Enzymes Are Examples Of Heterogeneous Catalyst homogeneous and heterogeneous catalysis: A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. As shown in part (a) in. Bridging the gap through surface organometallic chemistry.. Enzymes Are Examples Of Heterogeneous Catalyst.
From exovalyuk.blob.core.windows.net
Catalytic Group Definition at William Barham blog Enzymes Are Examples Of Heterogeneous Catalyst Such catalysts are usually solids, and often function by furnishing an active. Bridging the gap through surface organometallic chemistry. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. Catalysis is a phenomenon in which the rate of the reaction is altered. homogeneous and heterogeneous catalysis: an example. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.sliderbase.com
Catalysis Presentation Chemistry Enzymes Are Examples Of Heterogeneous Catalyst Bridging the gap through surface organometallic chemistry. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. As shown in part (a) in. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. an example of heterogeneous catalysis is using a. Enzymes Are Examples Of Heterogeneous Catalyst.
From slidetodoc.com
Immobilized Enzymes Reactors The methods for the heterogenisation Enzymes Are Examples Of Heterogeneous Catalyst Such catalysts are usually solids, and often function by furnishing an active. mechanism of an enzyme catalyst. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. As shown in part. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Heterogeneous catalysis PowerPoint Presentation, free download Enzymes Are Examples Of Heterogeneous Catalyst an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. As shown in part (a) in. Such catalysts are usually solids, and often function by furnishing an active. mechanism of an enzyme catalyst. Heterogeneous catalysts are catalysts that speed up the rate of reactions by. Enzymes Are Examples Of Heterogeneous Catalyst.
From en.ppt-online.org
Heterogeneous catalysis online presentation Enzymes Are Examples Of Heterogeneous Catalyst Bridging the gap through surface organometallic chemistry. mechanism of an enzyme catalyst. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. homogeneous and heterogeneous catalysis: A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid). Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Heterogeneous catalysis PowerPoint Presentation, free download Enzymes Are Examples Of Heterogeneous Catalyst A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. homogeneous and heterogeneous catalysis: an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. Catalysis is a phenomenon in which the rate of the reaction. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.researchgate.net
Comparison between heterogeneous, homogeneous, and nanocatalyst Enzymes Are Examples Of Heterogeneous Catalyst an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. As shown in part (a) in. Such catalysts are usually solids, and often function by furnishing an active. mechanism of an enzyme catalyst. a heterogeneous catalyst is present in a different phase. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID6835497 Enzymes Are Examples Of Heterogeneous Catalyst homogeneous and heterogeneous catalysis: Catalysis is a phenomenon in which the rate of the reaction is altered. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. As shown in part (a) in. a heterogeneous catalyst is present in a different phase from the. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Enzymes Are Examples Of Heterogeneous Catalyst mechanism of an enzyme catalyst. As shown in part (a) in. Such catalysts are usually solids, and often function by furnishing an active. homogeneous and heterogeneous catalysis: A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Enzymes Are Examples Of Heterogeneous Catalyst As shown in part (a) in. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or pt. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. a heterogeneous catalyst. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Enzymes Are Examples Of Heterogeneous Catalyst Such catalysts are usually solids, and often function by furnishing an active. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. Catalysis is a phenomenon in which the rate of the reaction is altered. mechanism of an enzyme catalyst. an example of heterogeneous catalysis is using a. Enzymes Are Examples Of Heterogeneous Catalyst.
From facts.net
18 Unbelievable Facts About Enzyme Catalysis Enzymes Are Examples Of Heterogeneous Catalyst Bridging the gap through surface organometallic chemistry. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. a heterogeneous catalyst is present in a different phase from the reactants. Catalysis is a phenomenon in which the rate of the reaction is altered. . Enzymes Are Examples Of Heterogeneous Catalyst.
From 2012books.lardbucket.org
Catalysis Enzymes Are Examples Of Heterogeneous Catalyst mechanism of an enzyme catalyst. As shown in part (a) in. Bridging the gap through surface organometallic chemistry. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface of a metal, such as ni, pd, or. Enzymes Are Examples Of Heterogeneous Catalyst.
From en.ppt-online.org
Heterogeneous catalysis online presentation Enzymes Are Examples Of Heterogeneous Catalyst A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. Such catalysts are usually solids, and often function by furnishing an active. a heterogeneous catalyst is present in a different phase from the reactants. mechanism of an enzyme catalyst. Catalysis is a phenomenon in which the rate of the reaction. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideserve.com
PPT Heterogeneous Catalysis 6 lectures PowerPoint Presentation, free Enzymes Are Examples Of Heterogeneous Catalyst Bridging the gap through surface organometallic chemistry. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. a heterogeneous catalyst is present in a different phase from the reactants. mechanism of an enzyme catalyst. Catalysis is a phenomenon in which the rate of the reaction is altered. As. Enzymes Are Examples Of Heterogeneous Catalyst.
From slidetodoc.com
Heterogeneous Catalysis Solid State Physics 141 A Dohyung Enzymes Are Examples Of Heterogeneous Catalyst Catalysis is a phenomenon in which the rate of the reaction is altered. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the. Heterogeneous catalysts are catalysts that speed up the rate of reactions by allowing them to occur on a solid surface. an example of heterogeneous catalysis is the interaction. Enzymes Are Examples Of Heterogeneous Catalyst.
From www.slideshare.net
Heterogeneous catalysisFundamentals Enzymes Are Examples Of Heterogeneous Catalyst Catalysis is a phenomenon in which the rate of the reaction is altered. mechanism of an enzyme catalyst. an example of heterogeneous catalysis is using a solid catalyst like a zeolite or alumina to catalyze a reaction in a mixture of liquids and/or. an example of heterogeneous catalysis is the interaction of hydrogen gas with the surface. Enzymes Are Examples Of Heterogeneous Catalyst.