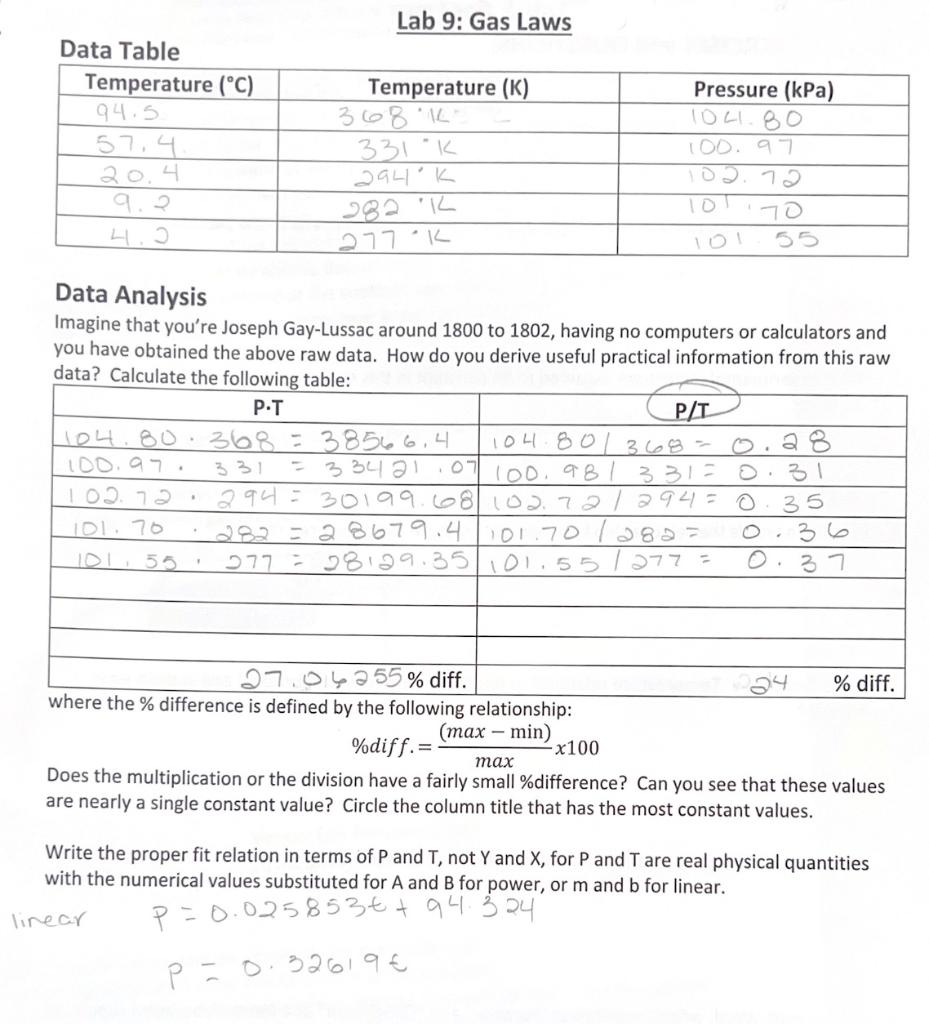
Lab 9 Gas Laws . Gases have been one of the most useful types of substances for driving scientific. It is derived from the following. The purpose of this experiment was to determine the molecular mass of an unknown. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. in this experiment, we explore gas laws; the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. lab 9 report experiment title: in part i, we will examine the relationship between the volume and pressure for a gas. Boyle’s and charles’ and the relationship pressure and volume have with gas.
from www.chegg.com
gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. in part i, we will examine the relationship between the volume and pressure for a gas. in this experiment, we explore gas laws; The purpose of this experiment was to determine the molecular mass of an unknown. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Boyle’s and charles’ and the relationship pressure and volume have with gas. It is derived from the following. lab 9 report experiment title: Gases have been one of the most useful types of substances for driving scientific.
Lab 9 Gas Laws PostLab EXERCISES and QUESTIONS 1.
Lab 9 Gas Laws in part i, we will examine the relationship between the volume and pressure for a gas. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. in this experiment, we explore gas laws; in part i, we will examine the relationship between the volume and pressure for a gas. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. Boyle’s and charles’ and the relationship pressure and volume have with gas. Gases have been one of the most useful types of substances for driving scientific. The purpose of this experiment was to determine the molecular mass of an unknown. lab 9 report experiment title: gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. It is derived from the following.
From www.studocu.com
Gas Laws Lab Gas Laws Lab, Fall Term 20192020 Experiment 2 Lab 9 Gas Laws It is derived from the following. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. lab 9 report experiment title: The purpose of this experiment was to determine the molecular mass of an unknown. in part i, we will examine the relationship between the volume. Lab 9 Gas Laws.
From www.chegg.com
Solved Experiment 9 Gas Law Molar Mass of a Volatile Liquid Lab 9 Gas Laws the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. The purpose of this experiment was to determine the molecular mass of an unknown. Boyle’s and charles’ and the relationship pressure and volume have with gas. these gas laws, now recognized as special cases of the the. Lab 9 Gas Laws.
From www.chegg.com
Solved Experiment 9 Gas Laws PRELABORATORY ASSIGNMENT This Lab 9 Gas Laws Boyle’s and charles’ and the relationship pressure and volume have with gas. in this experiment, we explore gas laws; Gases have been one of the most useful types of substances for driving scientific. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. gas laws are a group of. Lab 9 Gas Laws.
From www.chegg.com
Solved Lab 9 Gas Laws Data Table Volume (mL) 5.0 7.0 9.0 Lab 9 Gas Laws Boyle’s and charles’ and the relationship pressure and volume have with gas. Gases have been one of the most useful types of substances for driving scientific. gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. lab 9 report experiment title: The purpose of this experiment. Lab 9 Gas Laws.
From www.studocu.com
Gas Laws Lab Sheet lab Gas Laws Lab Sheet Sarah Carrasquillo, John Lab 9 Gas Laws lab 9 report experiment title: The purpose of this experiment was to determine the molecular mass of an unknown. in this experiment, we explore gas laws; gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. the ideal gas law utilises a variety of. Lab 9 Gas Laws.
From www.pinterest.com
This sheet gives the formulas for the Gas Laws. MA.912.HSAAPR.C Lab 9 Gas Laws gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. in this experiment, we explore gas laws; Boyle’s and charles’ and the relationship pressure and volume have with gas. Gases have been one of the most useful types of substances for driving scientific. The purpose of. Lab 9 Gas Laws.
From www.studocu.com
Exploring Gas Laws Lab Exploring Gas Laws Lab Data and Results A Lab 9 Gas Laws in this experiment, we explore gas laws; Gases have been one of the most useful types of substances for driving scientific. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Boyle’s and charles’ and the relationship pressure and volume have with gas. The purpose of this. Lab 9 Gas Laws.
From studylib.net
AP CHEM NOTES GAS LAWS Lab 9 Gas Laws these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. in part i, we will examine the relationship between the volume and pressure for a gas. lab 9 report experiment title: in this experiment, we explore gas laws; It is derived from the following. Boyle’s and charles’ and. Lab 9 Gas Laws.
From www.studocu.com
Key 8.1 Gas Law Lab Gas Lab key for chapter 8 information 8 Lab 9 Gas Laws in this experiment, we explore gas laws; Gases have been one of the most useful types of substances for driving scientific. The purpose of this experiment was to determine the molecular mass of an unknown. in part i, we will examine the relationship between the volume and pressure for a gas. these gas laws, now recognized as. Lab 9 Gas Laws.
From www.chegg.com
Solved Lab 9 Gas Laws in part i, we will examine the relationship between the volume and pressure for a gas. Boyle’s and charles’ and the relationship pressure and volume have with gas. Gases have been one of the most useful types of substances for driving scientific. gas laws are a group of laws that govern the behaviour of gases by providing relationships. Lab 9 Gas Laws.
From www.chegg.com
Solved Lab 9 Gas Laws Data Table Volume (mL) 5.0 7.0 9.0 Lab 9 Gas Laws the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. It is derived from the following. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. The purpose of this experiment was to determine the molecular mass of an. Lab 9 Gas Laws.
From maaphilross.blogspot.com
boyle's law experiment report Phil Ross Lab 9 Gas Laws The purpose of this experiment was to determine the molecular mass of an unknown. It is derived from the following. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. Gases have been one of the most useful types of substances for driving scientific. Boyle’s and charles’ and the relationship pressure. Lab 9 Gas Laws.
From www.studocu.com
Lab Report 9 Gas Laws Boyle’s and Charle’s Laws Basic Lab Report Lab 9 Gas Laws The purpose of this experiment was to determine the molecular mass of an unknown. It is derived from the following. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas. Lab 9 Gas Laws.
From www.chegg.com
Solved GAS LAW IDEAL GAS LAW CONSTANT INTRODUCTION Lab 9 Gas Laws The purpose of this experiment was to determine the molecular mass of an unknown. in part i, we will examine the relationship between the volume and pressure for a gas. lab 9 report experiment title: It is derived from the following. gas laws are a group of laws that govern the behaviour of gases by providing relationships. Lab 9 Gas Laws.
From www.studocu.com
Lab Report 9 Gas Laws 4/19/ Lab 9 Report Experiment Title Gas Laws Lab 9 Gas Laws Gases have been one of the most useful types of substances for driving scientific. Boyle’s and charles’ and the relationship pressure and volume have with gas. The purpose of this experiment was to determine the molecular mass of an unknown. gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature,. Lab 9 Gas Laws.
From www.scribd.com
Chem Lab Report 9Gas Law PDF Gases Temperature Lab 9 Gas Laws gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. in this experiment, we explore gas laws; It is derived from the following. Boyle’s. Lab 9 Gas Laws.
From www.studocu.com
Lab 9 Gas chromatography Title Gas Chromatography Introduction In Lab 9 Gas Laws The purpose of this experiment was to determine the molecular mass of an unknown. Boyle’s and charles’ and the relationship pressure and volume have with gas. Gases have been one of the most useful types of substances for driving scientific. in this experiment, we explore gas laws; the ideal gas law utilises a variety of observable gas properties. Lab 9 Gas Laws.
From www.studocu.com
Lab9 Gas Law assignment LAB9 Gas Law Post lab assignment 1) Graph Lab 9 Gas Laws It is derived from the following. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. in part i, we will examine the relationship between the volume and pressure for a gas. Gases have been one of the most useful types of substances for driving scientific. lab 9 report. Lab 9 Gas Laws.
From www.chegg.com
Solved Name Lab Instructor Date . Experiment 9 Gas Laws Lab 9 Gas Laws It is derived from the following. Boyle’s and charles’ and the relationship pressure and volume have with gas. in this experiment, we explore gas laws; The purpose of this experiment was to determine the molecular mass of an unknown. Gases have been one of the most useful types of substances for driving scientific. in part i, we will. Lab 9 Gas Laws.
From www.studocu.com
Lab 9 Evaluation of the Gas Law Constant CHEM 1411 LSC Studocu Lab 9 Gas Laws in part i, we will examine the relationship between the volume and pressure for a gas. Boyle’s and charles’ and the relationship pressure and volume have with gas. Gases have been one of the most useful types of substances for driving scientific. lab 9 report experiment title: It is derived from the following. these gas laws, now. Lab 9 Gas Laws.
From www.studocu.com
Bernardo Genchem Labexercise 9 LABORATORY EXERCISE 9 GAS LAWS General Lab 9 Gas Laws It is derived from the following. lab 9 report experiment title: Boyle’s and charles’ and the relationship pressure and volume have with gas. Gases have been one of the most useful types of substances for driving scientific. in this experiment, we explore gas laws; these gas laws, now recognized as special cases of the the ideal gas. Lab 9 Gas Laws.
From www.chegg.com
Solved Experiment 9 Gas Laws NAME Section Date Lab 9 Gas Laws in this experiment, we explore gas laws; It is derived from the following. lab 9 report experiment title: Gases have been one of the most useful types of substances for driving scientific. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. in part i,. Lab 9 Gas Laws.
From www.chegg.com
Solved Lab 9 Gas Laws and Molar Volume of Carbon Dioxide Lab 9 Gas Laws these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. Gases have been one of the most useful types of substances for driving scientific. in part i, we. Lab 9 Gas Laws.
From www.chegg.com
Solved Experiment 9 Gas Laws PRELABORATORY ASSIGNMENT This Lab 9 Gas Laws It is derived from the following. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. in part i, we will examine the relationship between the volume and pressure for a gas. Gases have been one of the most useful types of substances for driving scientific. The. Lab 9 Gas Laws.
From www.chegg.com
Lab 9 Gas Laws PostLab EXERCISES and QUESTIONS 1. Lab 9 Gas Laws gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. in this experiment, we explore gas laws; in part i, we will examine the relationship between the volume and pressure for a gas. The purpose of this experiment was to determine the molecular mass of. Lab 9 Gas Laws.
From www.studypool.com
SOLUTION CHM 113 Experiment 9 Gas Laws Lab Report Studypool Lab 9 Gas Laws Gases have been one of the most useful types of substances for driving scientific. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. The. Lab 9 Gas Laws.
From www.chegg.com
Virtual Lab 9 Gas Laws GAS LAW IDEAL GAS LAW Lab 9 Gas Laws lab 9 report experiment title: The purpose of this experiment was to determine the molecular mass of an unknown. Boyle’s and charles’ and the relationship pressure and volume have with gas. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. in part i, we will examine the relationship. Lab 9 Gas Laws.
From www.studypool.com
SOLUTION Chem 2211l lab 9 gas chromatography post lab Studypool Lab 9 Gas Laws It is derived from the following. in this experiment, we explore gas laws; Boyle’s and charles’ and the relationship pressure and volume have with gas. gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. lab 9 report experiment title: The purpose of this experiment. Lab 9 Gas Laws.
From www.chegg.com
Solved Gas Laws and Stoichiometry Prelab Prelab Calculations Lab 9 Gas Laws Boyle’s and charles’ and the relationship pressure and volume have with gas. in part i, we will examine the relationship between the volume and pressure for a gas. The purpose of this experiment was to determine the molecular mass of an unknown. the ideal gas law utilises a variety of observable gas properties to create one universal equation. Lab 9 Gas Laws.
From studylib.net
Gas Laws Lab Lab 9 Gas Laws in this experiment, we explore gas laws; It is derived from the following. the ideal gas law utilises a variety of observable gas properties to create one universal equation to model ideal gas behaviour. in part i, we will examine the relationship between the volume and pressure for a gas. Gases have been one of the most. Lab 9 Gas Laws.
From www.chegg.com
Lab 9 Gas Laws PostLab EXERCISES and QUESTIONS 1. Lab 9 Gas Laws in this experiment, we explore gas laws; Boyle’s and charles’ and the relationship pressure and volume have with gas. lab 9 report experiment title: The purpose of this experiment was to determine the molecular mass of an unknown. It is derived from the following. gas laws are a group of laws that govern the behaviour of gases. Lab 9 Gas Laws.
From www.scribd.com
Chem Lab Report 9 (2)Gas Law Gases Temperature Lab 9 Gas Laws Boyle’s and charles’ and the relationship pressure and volume have with gas. gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. Gases have been one of the most useful types of substances for driving scientific. The purpose of this experiment was to determine the molecular mass. Lab 9 Gas Laws.
From studylib.net
Gas Laws Gizmo Lab Lab 9 Gas Laws lab 9 report experiment title: The purpose of this experiment was to determine the molecular mass of an unknown. Gases have been one of the most useful types of substances for driving scientific. in this experiment, we explore gas laws; Boyle’s and charles’ and the relationship pressure and volume have with gas. gas laws are a group. Lab 9 Gas Laws.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Lab 9 Gas Laws It is derived from the following. Gases have been one of the most useful types of substances for driving scientific. gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and. The purpose of this experiment was to determine the molecular mass of an unknown. Boyle’s and charles’. Lab 9 Gas Laws.
From www.studypool.com
SOLUTION CHM 113 Experiment 9 Gas Laws Lab Report Studypool Lab 9 Gas Laws The purpose of this experiment was to determine the molecular mass of an unknown. in this experiment, we explore gas laws; Gases have been one of the most useful types of substances for driving scientific. these gas laws, now recognized as special cases of the the ideal gas equation, describe the effect of. It is derived from the. Lab 9 Gas Laws.