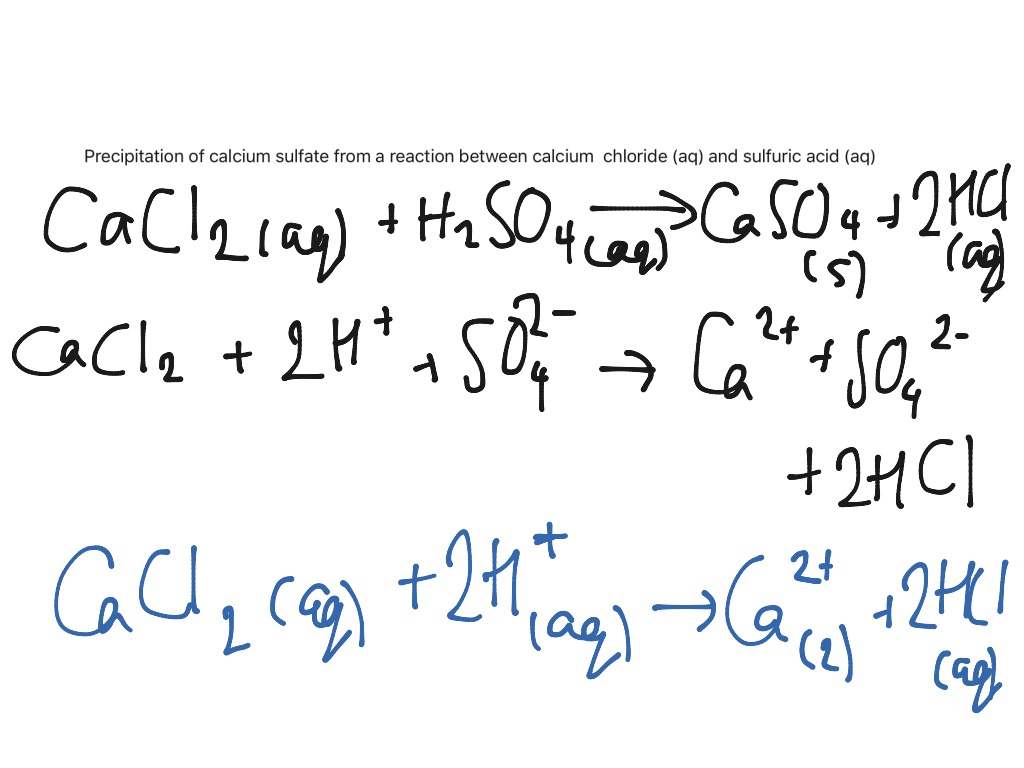Magnesium Chloride Precipitate Equation . Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. Enter an equation of an ionic chemical equation and press the balance button. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: Many reactions of this type involve. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The balanced equation will be calculated along with the. While full chemical equations show the. Some of the solid agcl continues to dissolve, but at the same time,.
from mungfali.com
Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Some of the solid agcl continues to dissolve, but at the same time,. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. The balanced equation will be calculated along with the. Many reactions of this type involve. Enter an equation of an ionic chemical equation and press the balance button. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). While full chemical equations show the.
Calcium Chloride And Water Reaction
Magnesium Chloride Precipitate Equation Many reactions of this type involve. Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: Many reactions of this type involve. The balanced equation will be calculated along with the. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). While full chemical equations show the. Enter an equation of an ionic chemical equation and press the balance button. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. Some of the solid agcl continues to dissolve, but at the same time,.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium Chloride Precipitate Equation Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring. Magnesium Chloride Precipitate Equation.
From www.tessshebaylo.com
Balanced Equation For Dissolving Ammonium Chloride In Water Tessshebaylo Magnesium Chloride Precipitate Equation While full chemical equations show the. The balanced equation will be calculated along with the. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: Enter an equation of an ionic chemical equation and press the balance button. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). Agcl(s) dissolution ⇌. Magnesium Chloride Precipitate Equation.
From armsingle10.pythonanywhere.com
Supreme Mgcl2 And Water Equation All Formulas Of Science Class 9 Ncert Magnesium Chloride Precipitate Equation Many reactions of this type involve. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. The balanced equation will be calculated along with the. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED An aqueous solution of magnesium chloride, MgCl2, is added to Magnesium Chloride Precipitate Equation Many reactions of this type involve. While full chemical equations show the. Some of the solid agcl continues to dissolve, but at the same time,. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in. Magnesium Chloride Precipitate Equation.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride Precipitate Equation While full chemical equations show the. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; If a precipitate is formed. Magnesium Chloride Precipitate Equation.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium Chloride Precipitate Equation A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Enter an equation of an ionic chemical equation and press the balance button. Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED'Write balanced equalion for the reaction _ Sodium hydroxide is Magnesium Chloride Precipitate Equation Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. Enter an equation of an ionic chemical equation and press the. Magnesium Chloride Precipitate Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride Word Equation Magnesium Chloride Precipitate Equation If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. Some of the solid agcl continues to dissolve, but at the same time,. Many reactions of this type involve. Enter an equation of an ionic chemical equation and press. Magnesium Chloride Precipitate Equation.
From www.toppr.com
Magnesium combines with chlorine to form magnesium chloride. The Magnesium Chloride Precipitate Equation The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: Enter an equation of an ionic chemical equation and press the balance button. Some of the solid agcl continues to dissolve, but at the same time,. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). A precipitation reaction is one. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED How many formula units make up 20.0 gg of magnesium chloride Magnesium Chloride Precipitate Equation Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; The balanced equation will be calculated along with the. Some of the solid agcl continues to dissolve, but at the same time,. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. If a precipitate. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Magnesium Chloride Precipitate Equation Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; The balanced equation will be calculated along with the. If a. Magnesium Chloride Precipitate Equation.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Magnesium Chloride Precipitate Equation Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). While full chemical equations show the. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. Many reactions of this type involve. Enter an equation. Magnesium Chloride Precipitate Equation.
From www.shalom-education.com
Testing for Sulfate Ions GCSE Chemistry Revision Magnesium Chloride Precipitate Equation Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: While full chemical equations show the. If a precipitate. Magnesium Chloride Precipitate Equation.
From www.nagwa.com
Question Video Calculating the Mass of the Magnesium Sulfate Analyte Magnesium Chloride Precipitate Equation The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: While full chemical equations show the. The balanced equation will be calculated along with the. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). If a precipitate is formed when a chemical reacts with lead, for example, the presence of. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED When solutions of silver nitrate and magnesium chloride are Magnesium Chloride Precipitate Equation While full chemical equations show the. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Some of the solid agcl continues to dissolve, but at the same time,. Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; Enter an equation of an ionic. Magnesium Chloride Precipitate Equation.
From animalia-life.club
Magnesium Sulfate Lewis Structure Magnesium Chloride Precipitate Equation The balanced equation will be calculated along with the. While full chemical equations show the. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Some of the solid agcl continues to dissolve, but at the same time,. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is:. Magnesium Chloride Precipitate Equation.
From www.youtube.com
Empirical Formula of Magnesium Chloride LAB YouTube Magnesium Chloride Precipitate Equation Some of the solid agcl continues to dissolve, but at the same time,. Many reactions of this type involve. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. Enter an equation of an ionic chemical equation and press. Magnesium Chloride Precipitate Equation.
From www.coursehero.com
[Solved] 6,881 g of a sample containing magnesium chloride and sodium Magnesium Chloride Precipitate Equation Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. The equation for the equilibrium between solid silver chloride, silver ion,. Magnesium Chloride Precipitate Equation.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium Chloride Precipitate Equation A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. The balanced equation will be calculated along with the. Mixing potassium chromate. Magnesium Chloride Precipitate Equation.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride Precipitate Equation The balanced equation will be calculated along with the. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; Many reactions of this. Magnesium Chloride Precipitate Equation.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride Precipitate Equation While full chemical equations show the. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: A precipitation reaction. Magnesium Chloride Precipitate Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Magnesium Chloride Precipitate Equation Many reactions of this type involve. While full chemical equations show the. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. The balanced equation will be calculated along with the. Mixing potassium chromate and silver nitrate together to. Magnesium Chloride Precipitate Equation.
From www.youtube.com
How to Write the Formula for Magnesium chlorate YouTube Magnesium Chloride Precipitate Equation Many reactions of this type involve. While full chemical equations show the. Enter an equation of an ionic chemical equation and press the balance button. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: A precipitation reaction is one in. Magnesium Chloride Precipitate Equation.
From www.youtube.com
NaOH + MgSO4 Reaction & Precipitate Blossoms 💮 YouTube Magnesium Chloride Precipitate Equation Many reactions of this type involve. Some of the solid agcl continues to dissolve, but at the same time,. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The balanced equation will be calculated along with the. Agcl(s). Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED Consider the double replacement reactions The reaction of Magnesium Chloride Precipitate Equation Many reactions of this type involve. Some of the solid agcl continues to dissolve, but at the same time,. While full chemical equations show the. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; The balanced equation. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED 3e. Magnesium Chloride and Sodium Carbonate Was a precipitate Magnesium Chloride Precipitate Equation While full chemical equations show the. Enter an equation of an ionic chemical equation and press the balance button. Some of the solid agcl continues to dissolve, but at the same time,. The balanced equation will be calculated along with the. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Mixing potassium. Magnesium Chloride Precipitate Equation.
From mungfali.com
Calcium Chloride And Water Reaction Magnesium Chloride Precipitate Equation The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). The balanced equation will be calculated along with the. Agcl(s) dissolution ⇌ precipitation ag. Magnesium Chloride Precipitate Equation.
From ar.inspiredpencil.com
Reactants In A Chemical Equation Magnesium Chloride Precipitate Equation The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: Many reactions of this type involve. Enter an equation of an ionic chemical equation and press the balance button. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED3e. Magnesium Chloride and Sodium Carbonate Was a precipitate Magnesium Chloride Precipitate Equation If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; The equation for the equilibrium between solid silver chloride, silver ion,. Magnesium Chloride Precipitate Equation.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Precipitate Equation Enter an equation of an ionic chemical equation and press the balance button. Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; Some of the solid agcl continues to dissolve, but at the same time,. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: If. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED Consider the following experiment What is a possible identity Magnesium Chloride Precipitate Equation Enter an equation of an ionic chemical equation and press the balance button. Many reactions of this type involve. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. The equation for the equilibrium between solid silver chloride, silver. Magnesium Chloride Precipitate Equation.
From loetrsxww.blob.core.windows.net
Hydrolysis Of Iron(Iii) Chloride Equation at Lamphere blog Magnesium Chloride Precipitate Equation Agcl(s) dissolution ⇌ precipitation ag + (aq) + cl − (aq) this equilibrium, like other equilibria, is dynamic; A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation \(\ref{4.2.1}\)). If a precipitate is formed when a chemical reacts with lead,. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVEDWrite net ionic equations for the formation of (a) a precipitate Magnesium Chloride Precipitate Equation While full chemical equations show the. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate. A precipitation reaction is one in which dissolved. Magnesium Chloride Precipitate Equation.
From cerzmopv.blob.core.windows.net
Magnesium And Zinc Nitrate Equation at Terry Ranson blog Magnesium Chloride Precipitate Equation The balanced equation will be calculated along with the. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water. Magnesium Chloride Precipitate Equation.
From www.numerade.com
SOLVED 1.78 g magnesium chloride is mixed with 2.50 g potassium Magnesium Chloride Precipitate Equation Some of the solid agcl continues to dissolve, but at the same time,. The equation for the equilibrium between solid silver chloride, silver ion, and chloride ion is: Many reactions of this type involve. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. The balanced equation will be calculated along with the.. Magnesium Chloride Precipitate Equation.