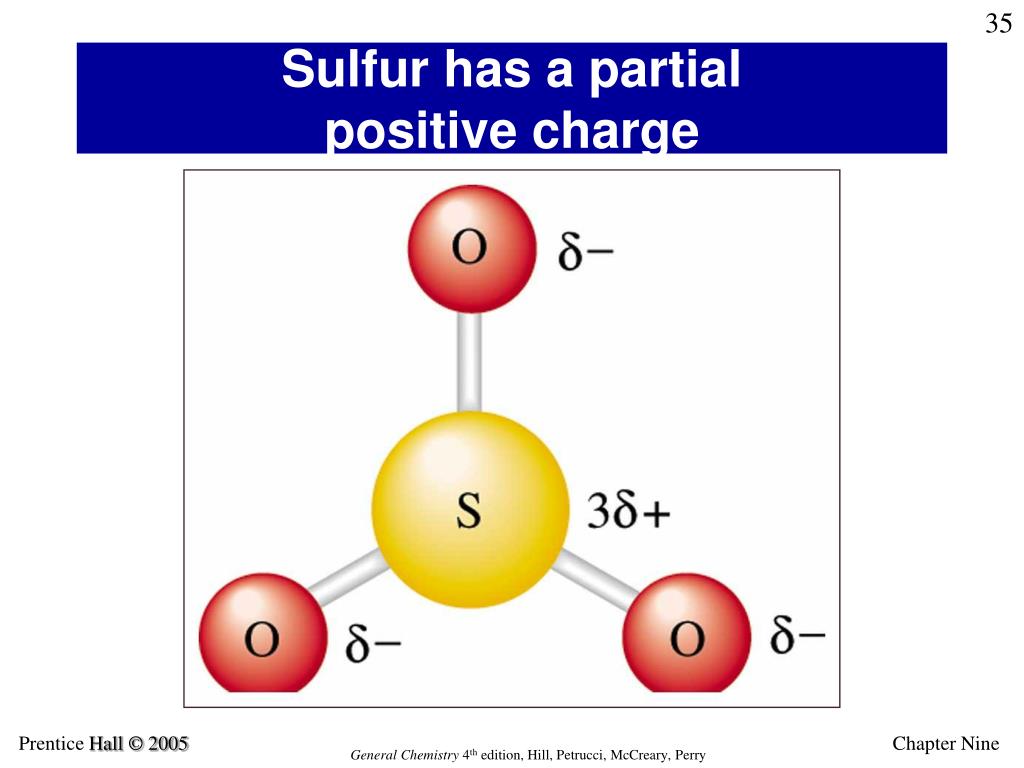
Does Sulfur Form A Positive Ion . A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. In this state, it does not have a net. Most other metals form cations (e.g. Iron, silver, nickel), whilst most other nonmetals.
from www.slideserve.com
Iron, silver, nickel), whilst most other nonmetals. In this state, it does not have a net. Most other metals form cations (e.g. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when.
PPT Chemical Bonds PowerPoint Presentation, free download ID4438872
Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. In this state, it does not have a net. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Iron, silver, nickel), whilst most other nonmetals. Most other metals form cations (e.g.
From explainopedia.com
Is Sulfur Positive or Negative? Its Charge and Role in Chemistry Does Sulfur Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Most other metals form cations (e.g. In this state, it does not. Does Sulfur Form A Positive Ion.
From elchoroukhost.net
Periodic Table Sulfur Protons Neutrons Electrons Elcho Table Does Sulfur Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Most other metals form cations (e.g. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. In this state, it does. Does Sulfur Form A Positive Ion.
From sites.google.com
Sulfur Table of Elements by Shrenil Sharma Does Sulfur Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Iron, silver, nickel), whilst most other nonmetals. In this state, it does not have a net. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Sulfur is an atom,. Does Sulfur Form A Positive Ion.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID4438872 Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Iron, silver, nickel), whilst most other nonmetals. In this state, it does not have a net. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. Atoms that lose. Does Sulfur Form A Positive Ion.
From material-properties.org
Sulfur Periodic Table and Atomic Properties Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Halogens always form anions, alkali metals and alkaline earth metals always form cations. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion). Does Sulfur Form A Positive Ion.
From periodictable.me
Sulfur Electron Configuration (S) with Orbital Diagram Does Sulfur Form A Positive Ion Halogens always form anions, alkali metals and alkaline earth metals always form cations. Iron, silver, nickel), whilst most other nonmetals. In this state, it does not have a net. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Atoms that lose electrons acquire a positive charge because. Does Sulfur Form A Positive Ion.
From www.youtube.com
How to Find the Ionic Charge for Sulfur (S) YouTube Does Sulfur Form A Positive Ion In this state, it does not have a net. Iron, silver, nickel), whilst most other nonmetals. Most other metals form cations (e.g. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms that lose electrons acquire a positive charge because they are. Does Sulfur Form A Positive Ion.
From www.nagwa.com
Question Video Identifying Which Element Can Form a Positive Ion in an Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Does Sulfur Form A Positive Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Sulfur Form A Positive Ion Iron, silver, nickel), whilst most other nonmetals. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Halogens always form anions, alkali metals and alkaline earth metals always form cations. In this state, it does not have a net. Sulfur is an atom, and as an element, it exists. Does Sulfur Form A Positive Ion.
From www.nuclear-power.com
Sulfur Electron Affinity Electronegativity Ionization Energy of Does Sulfur Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Iron, silver, nickel), whilst most other nonmetals. A cation (a positive ion) forms when a neutral atom. Does Sulfur Form A Positive Ion.
From www.numerade.com
SOLVEDThe orbital notation of sulfur is shown in Figure 7.15. Explain Does Sulfur Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Halogens always form anions, alkali metals and alkaline earth metals always form. Does Sulfur Form A Positive Ion.
From wirelistlatinised.z21.web.core.windows.net
So2 Phase Diagram Does Sulfur Form A Positive Ion Iron, silver, nickel), whilst most other nonmetals. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. In this state, it does not have a net. Atoms that lose electrons acquire a positive charge because. Does Sulfur Form A Positive Ion.
From sciencenotes.org
Sulfur Atom Science Notes and Projects Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In this state, it. Does Sulfur Form A Positive Ion.
From ar.inspiredpencil.com
Electron Configuration Of Sulfur Does Sulfur Form A Positive Ion In this state, it does not have a net. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Iron, silver, nickel), whilst most other nonmetals. Sulfur is an atom, and as an element, it exists in a neutral state with an equal. Does Sulfur Form A Positive Ion.
From periodictable.me
Sulfur Electron Configuration (S) with Orbital Diagram Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms that lose electrons acquire a positive charge because they are. Does Sulfur Form A Positive Ion.
From worksheetzonejimply.z5.web.core.windows.net
Chemistry Positive And Negative Ions Does Sulfur Form A Positive Ion In this state, it does not have a net. Iron, silver, nickel), whilst most other nonmetals. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Halogens always form anions, alkali metals and alkaline earth metals always form cations. A cation (a positive ion) forms when a neutral atom. Does Sulfur Form A Positive Ion.
From stock.adobe.com
Sulfur atomic structure has atomic number, atomic mass, electron Does Sulfur Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Iron, silver, nickel), whilst most other nonmetals. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Halogens always form anions, alkali. Does Sulfur Form A Positive Ion.
From utedzz.blogspot.com
Periodic Table Sulfur Ion Periodic Table Timeline Does Sulfur Form A Positive Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Sulfur is an atom, and as an element, it exists in a. Does Sulfur Form A Positive Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Does Sulfur Form A Positive Ion Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms. Does Sulfur Form A Positive Ion.
From utedzz.blogspot.com
Periodic Table Sulfur Ion Periodic Table Timeline Does Sulfur Form A Positive Ion Most other metals form cations (e.g. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In this state, it does not have a net. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Halogens always. Does Sulfur Form A Positive Ion.
From slideplayer.com
Chemical Nomenclature ppt download Does Sulfur Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Sulfur is an atom, and as an element, it exists in a. Does Sulfur Form A Positive Ion.
From www.slideserve.com
PPT Formulas Review PowerPoint Presentation, free download ID2524281 Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Most other metals form cations (e.g. In this state, it does not have a net. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Halogens always. Does Sulfur Form A Positive Ion.
From ar.inspiredpencil.com
Sulfur Protons Neutrons Electrons Does Sulfur Form A Positive Ion Halogens always form anions, alkali metals and alkaline earth metals always form cations. Iron, silver, nickel), whilst most other nonmetals. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In this state, it does not have a net. Sulfur is an atom, and as an element, it exists. Does Sulfur Form A Positive Ion.
From www.slideserve.com
PPT Chemical Names of Ionic Compounds PowerPoint Presentation, free Does Sulfur Form A Positive Ion In this state, it does not have a net. Iron, silver, nickel), whilst most other nonmetals. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Halogens always form anions, alkali metals and alkaline earth metals always form cations. A cation (a positive ion) forms when a neutral. Does Sulfur Form A Positive Ion.
From www.alamy.com
S Sulfur, Periodic Table of the Elements, Shell Structure of Sulfur Does Sulfur Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Iron, silver, nickel), whilst most other nonmetals. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Most other metals form cations. Does Sulfur Form A Positive Ion.
From mungfali.com
Valence Electrons Of Sulfur Does Sulfur Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Most other metals form cations (e.g. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. In this state, it does not. Does Sulfur Form A Positive Ion.
From www.vectorstock.com
Diagram representation of the element sulfur Vector Image Does Sulfur Form A Positive Ion In this state, it does not have a net. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because they are. Does Sulfur Form A Positive Ion.
From www.americanelements.com
Sulfur (S) AMERICAN ELEMENTS Does Sulfur Form A Positive Ion In this state, it does not have a net. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. Sulfur is an atom, and as an element, it exists in. Does Sulfur Form A Positive Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Iron, silver, nickel), whilst most other nonmetals. In this state, it does not have a net. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because. Does Sulfur Form A Positive Ion.
From www.vrogue.co
Chemistry 3 Draw The Atomic Structure Of A Sulfur Ato vrogue.co Does Sulfur Form A Positive Ion Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. In this state, it does not have a net. Iron, silver, nickel), whilst most other nonmetals. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Atoms that lose. Does Sulfur Form A Positive Ion.
From www.dreamstime.com
Sulfur Form Periodic Table Of Elements Stock Illustration Image 6508066 Does Sulfur Form A Positive Ion Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Most other metals form cations (e.g. Iron, silver, nickel), whilst most other nonmetals. Sulfur is an atom, and as an element, it exists in a neutral. Does Sulfur Form A Positive Ion.
From www.breakingatom.com
Ionic Bonding Does Sulfur Form A Positive Ion Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In this state, it does not have a net. Most other metals form cations (e.g. Iron, silver, nickel), whilst most other nonmetals. Sulfur is an atom,. Does Sulfur Form A Positive Ion.
From elchoroukhost.net
Periodic Table Sulfur Number Of Protons Elcho Table Does Sulfur Form A Positive Ion Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Iron, silver, nickel), whilst most other nonmetals. A cation (a positive ion) forms when a neutral atom loses one. Does Sulfur Form A Positive Ion.
From newtondesk.com
Sulfur S (Element 16) of Periodic Table Elements FlashCards Does Sulfur Form A Positive Ion Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. Iron, silver, nickel), whilst most other nonmetals. A cation (a positive ion) forms when a neutral atom loses one or. Does Sulfur Form A Positive Ion.
From encyclopedia.pub
Influence of Sulfur on the Origin of Life Encyclopedia MDPI Does Sulfur Form A Positive Ion Sulfur is an atom, and as an element, it exists in a neutral state with an equal number of protons and electrons. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (a positive. Does Sulfur Form A Positive Ion.