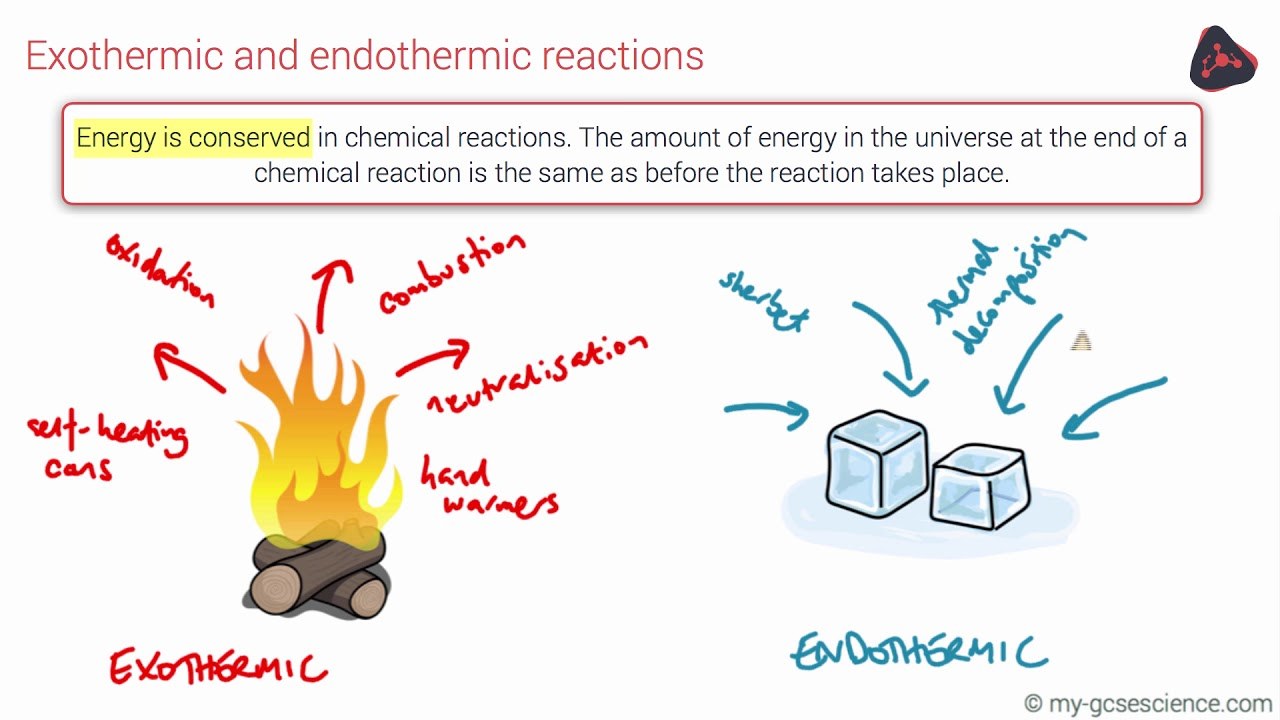
Endothermic Reaction Examples Ks3 . Some uses of exothermic reactions are: An endothermic chemical reaction causes a. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Examples of endothermic reactions include: When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. These reactions can also be. Endothermic reactions take in energy from their surroundings, usually in the form of heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Electrolysis the decomposition (breakdown) of a compound using an electric. Some examples of endothermic reactions are: Thermal decomposition type of reaction in which a compound breaks down to form two.
from www.animalia-life.club
An endothermic chemical reaction causes a. Some uses of exothermic reactions are: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Thermal decomposition type of reaction in which a compound breaks down to form two. Electrolysis the decomposition (breakdown) of a compound using an electric. These reactions can also be. Some examples of endothermic reactions are: Endothermic reactions take in energy from their surroundings, usually in the form of heat.
Endothermic Reaction Examples For Kids
Endothermic Reaction Examples Ks3 Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. These reactions can also be. Examples of endothermic reactions include: An endothermic chemical reaction causes a. Endothermic reactions take in energy from their surroundings, usually in the form of heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some uses of exothermic reactions are: When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Electrolysis the decomposition (breakdown) of a compound using an electric. Thermal decomposition type of reaction in which a compound breaks down to form two. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Some examples of endothermic reactions are:
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Examples Ks3 Endothermic reactions take in energy from their surroundings, usually in the form of heat. Examples of endothermic reactions include: Some examples of endothermic reactions are: An exothermic chemical reaction causes an increase in temperature (of the surroundings). When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Electrolysis the decomposition (breakdown) of. Endothermic Reaction Examples Ks3.
From www.youtube.com
Endothermic Reaction with examples YouTube Endothermic Reaction Examples Ks3 These reactions can also be. Some examples of endothermic reactions are: Some uses of exothermic reactions are: Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Thermal decomposition type of reaction in which a compound breaks down to form two. Electrolysis the decomposition (breakdown) of a compound using an electric. Endothermic reactions. Endothermic Reaction Examples Ks3.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Examples Ks3 Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Endothermic reactions take in energy from their surroundings, usually in the form of heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Thermal decomposition type of reaction in which a compound breaks down to form two. Some examples of endothermic reactions are: Some uses. Endothermic Reaction Examples Ks3.
From www.tes.com
6.4.1 Exothermic and endothermic reactions (AQA KS3 Activate 2 Endothermic Reaction Examples Ks3 When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. An endothermic chemical reaction causes a. Electrolysis the decomposition (breakdown) of a compound using an electric. Examples of endothermic reactions include: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Thermal decomposition type of reaction in which a compound. Endothermic Reaction Examples Ks3.
From www.shalom-education.com
Exothermic and Endothermic Reactions KS3 Chemistry Revision Endothermic Reaction Examples Ks3 An exothermic chemical reaction causes an increase in temperature (of the surroundings). Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Electrolysis the decomposition (breakdown) of a compound using an electric. Thermal decomposition type of reaction in which a compound breaks down to form two. Endothermic reactions absorb energy, resulting in a. Endothermic Reaction Examples Ks3.
From www.pinterest.ph
Endothermic and Exothermic Reactions infographic diagram showing Endothermic Reaction Examples Ks3 An exothermic chemical reaction causes an increase in temperature (of the surroundings). An endothermic chemical reaction causes a. Some uses of exothermic reactions are: Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. These reactions can also be. Thermal decomposition type of reaction in which a compound breaks down to form two.. Endothermic Reaction Examples Ks3.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Examples Ks3 When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Endothermic reactions take in energy from their surroundings, usually in the form of heat. Examples of endothermic reactions include: An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some uses of exothermic reactions are: Electrolysis the decomposition (breakdown) of. Endothermic Reaction Examples Ks3.
From animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Ks3 Electrolysis the decomposition (breakdown) of a compound using an electric. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some examples of endothermic reactions are: Examples of endothermic reactions include: Endothermic reactions take in energy from their surroundings, usually in. Endothermic Reaction Examples Ks3.
From www.tes.com
KS3 Year 7 Exothermic and Endothermic Reactions Teaching Resources Endothermic Reaction Examples Ks3 An endothermic chemical reaction causes a. Electrolysis the decomposition (breakdown) of a compound using an electric. Some examples of endothermic reactions are: Endothermic reactions take in energy from their surroundings, usually in the form of heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some uses of exothermic reactions are: Endothermic reactions absorb energy, resulting in. Endothermic Reaction Examples Ks3.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Examples Ks3 Endothermic reactions take in energy from their surroundings, usually in the form of heat. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Some examples of endothermic reactions are: An endothermic chemical reaction causes a. Thermal decomposition type of reaction in which a compound breaks down to form two. Examples of endothermic. Endothermic Reaction Examples Ks3.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Ks3 These reactions can also be. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Endothermic reactions take in energy from their surroundings, usually in the form of heat. An endothermic chemical reaction causes a. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Thermal decomposition type of reaction in. Endothermic Reaction Examples Ks3.
From www.shalom-education.com
Exothermic and Endothermic Reactions KS3 Chemistry Revision Endothermic Reaction Examples Ks3 Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Some examples of endothermic reactions are: An endothermic chemical reaction causes a. When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Examples of endothermic reactions include: Endothermic reactions take in energy from their surroundings,. Endothermic Reaction Examples Ks3.
From www.tes.com
Exothermic and Endothermic KS3 Activate Science Teaching Resources Endothermic Reaction Examples Ks3 Endothermic reactions take in energy from their surroundings, usually in the form of heat. An endothermic chemical reaction causes a. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Some examples of endothermic reactions are: An exothermic chemical reaction causes. Endothermic Reaction Examples Ks3.
From www.bharatagritech.com
Endothermic Reaction Examples, 51 OFF Endothermic Reaction Examples Ks3 An endothermic chemical reaction causes a. When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Thermal decomposition type of reaction in which a compound breaks down to form two. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Examples of endothermic reactions include: Some examples of endothermic reactions. Endothermic Reaction Examples Ks3.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Examples Ks3 An endothermic chemical reaction causes a. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Some uses of exothermic reactions are: Examples of endothermic reactions include: An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some examples of endothermic reactions are: Endothermic reactions take in energy from their surroundings, usually in the form of. Endothermic Reaction Examples Ks3.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Examples Ks3 Some uses of exothermic reactions are: Thermal decomposition type of reaction in which a compound breaks down to form two. Electrolysis the decomposition (breakdown) of a compound using an electric. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Endothermic reactions take in energy from their surroundings, usually in the form of heat. Some examples of endothermic. Endothermic Reaction Examples Ks3.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Ks3 These reactions can also be. An endothermic chemical reaction causes a. Endothermic reactions take in energy from their surroundings, usually in the form of heat. When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Some examples of endothermic reactions are: An exothermic chemical reaction causes an increase in temperature (of the. Endothermic Reaction Examples Ks3.
From stock.adobe.com
Endothermic reactions list with external energy outline collection set Endothermic Reaction Examples Ks3 Examples of endothermic reactions include: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Some examples of endothermic reactions are: Endothermic reactions take in energy from their surroundings, usually in the form of heat. Electrolysis the decomposition (breakdown) of a. Endothermic Reaction Examples Ks3.
From www.youtube.com
KS3 Exothermic and Endothermic Reactions YouTube Endothermic Reaction Examples Ks3 Some uses of exothermic reactions are: Electrolysis the decomposition (breakdown) of a compound using an electric. Examples of endothermic reactions include: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. An exothermic chemical reaction causes an increase in temperature (of the surroundings). These reactions can also be. An endothermic chemical reaction causes a. When energy is taken. Endothermic Reaction Examples Ks3.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Examples Ks3 Some examples of endothermic reactions are: Some uses of exothermic reactions are: An endothermic chemical reaction causes a. Endothermic reactions take in energy from their surroundings, usually in the form of heat. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Examples of endothermic reactions include: These reactions can also be. Thermal. Endothermic Reaction Examples Ks3.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Examples Ks3 An exothermic chemical reaction causes an increase in temperature (of the surroundings). These reactions can also be. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. An endothermic chemical reaction causes a. Some uses of exothermic reactions are: Some examples of endothermic reactions are: Endothermic reactions take in energy from their surroundings,. Endothermic Reaction Examples Ks3.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Examples Ks3 Examples of endothermic reactions include: Electrolysis the decomposition (breakdown) of a compound using an electric. These reactions can also be. When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Endothermic reactions take in energy from their surroundings, usually in. Endothermic Reaction Examples Ks3.
From studylib.net
Endothermic and Exothermic reaction Worksheet answers Endothermic Reaction Examples Ks3 Some examples of endothermic reactions are: Electrolysis the decomposition (breakdown) of a compound using an electric. An endothermic chemical reaction causes a. Examples of endothermic reactions include: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Endothermic reactions take. Endothermic Reaction Examples Ks3.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Examples Ks3 Electrolysis the decomposition (breakdown) of a compound using an electric. Examples of endothermic reactions include: Endothermic reactions take in energy from their surroundings, usually in the form of heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some uses of exothermic reactions are: When energy is taken in from the surroundings, this is called an endothermic. Endothermic Reaction Examples Ks3.
From scienceinfo.com
Endothermic Reaction with Interesting Examples Endothermic Reaction Examples Ks3 These reactions can also be. Thermal decomposition type of reaction in which a compound breaks down to form two. Examples of endothermic reactions include: Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Endothermic reactions take in energy from their. Endothermic Reaction Examples Ks3.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Ks3 Some examples of endothermic reactions are: Endothermic reactions take in energy from their surroundings, usually in the form of heat. Some uses of exothermic reactions are: Electrolysis the decomposition (breakdown) of a compound using an electric. Thermal decomposition type of reaction in which a compound breaks down to form two. An exothermic chemical reaction causes an increase in temperature (of. Endothermic Reaction Examples Ks3.
From mungfali.com
Endothermic Vs Exothermic Reactions Examples Endothermic Reaction Examples Ks3 When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Thermal decomposition type of reaction in which a compound breaks down to form two. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Some uses of exothermic reactions are: An exothermic chemical reaction causes. Endothermic Reaction Examples Ks3.
From www.tes.com
Endothermic and Exothermic reactions (lab based worksheet, KS3 Endothermic Reaction Examples Ks3 Thermal decomposition type of reaction in which a compound breaks down to form two. These reactions can also be. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. An endothermic chemical reaction causes a. Some uses of exothermic reactions are: When energy is taken in from the surroundings, this is called an. Endothermic Reaction Examples Ks3.
From animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Ks3 These reactions can also be. Endothermic reactions take in energy from their surroundings, usually in the form of heat. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature.. Endothermic Reaction Examples Ks3.
From www.doubtnut.com
Distinguish between Endothermic reaction and Exothermic reactions. Endothermic Reaction Examples Ks3 Endothermic reactions take in energy from their surroundings, usually in the form of heat. An endothermic chemical reaction causes a. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. These reactions can also be. Thermal decomposition type of reaction in which a compound breaks down to form two. Electrolysis the decomposition (breakdown). Endothermic Reaction Examples Ks3.
From www.tes.com
Exothermic and Endothermic Reactions Teaching Resources Endothermic Reaction Examples Ks3 These reactions can also be. Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Some uses of exothermic reactions are: Some examples of endothermic reactions are: Endothermic reactions take in energy from their surroundings, usually in the form of heat. An endothermic chemical reaction causes a. Thermal decomposition type of reaction in. Endothermic Reaction Examples Ks3.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Ks3 Examples of endothermic reactions include: Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. These reactions can also be. Electrolysis the decomposition (breakdown) of a compound using an electric. An endothermic chemical reaction causes a. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Endothermic reactions take in energy. Endothermic Reaction Examples Ks3.
From www.tes.com
Exothermic/Endothermic Reactions Worksheet and Teacher Sheet Teaching Endothermic Reaction Examples Ks3 Some examples of endothermic reactions are: An endothermic chemical reaction causes a. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Electrolysis the decomposition (breakdown) of a compound using an electric. Examples of endothermic reactions include: Thermal decomposition type of. Endothermic Reaction Examples Ks3.
From www.youtube.com
Endothermic & Exothermic reaction class 10 chemical reaction and Endothermic Reaction Examples Ks3 When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold. Endothermic reactions take in energy from their surroundings, usually in the form of heat. Some uses of exothermic reactions are: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. An exothermic chemical reaction causes an increase in temperature (of. Endothermic Reaction Examples Ks3.
From www.tes.com
KS3 Chemistry AQA C2 2 2 Comparing Endothermic and Exothermic Changes Endothermic Reaction Examples Ks3 Complete the energy profile diagram and state whether the reaction is endothermic or exothermic, explain your answer. Some examples of endothermic reactions are: Thermal decomposition type of reaction in which a compound breaks down to form two. Endothermic reactions take in energy from their surroundings, usually in the form of heat. These reactions can also be. Some uses of exothermic. Endothermic Reaction Examples Ks3.