Magnesium + Zinc Chloride Equation . The following equation describes a reaction between magnesium and zinc chloride: Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; Which of the following equations correctly. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Let's balance this equation using the inspection method. Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. Acid + metal → salt + hydrogen. First, we set all coefficients to 1: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. To balance a chemical equation,. Hydrochloric acid + zinc → zinc chloride +. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii).
from www.numerade.com
Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Which of the following equations correctly. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. The following equation describes a reaction between magnesium and zinc chloride: Let's balance this equation using the inspection method. Hydrochloric acid + zinc → zinc chloride +. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s).
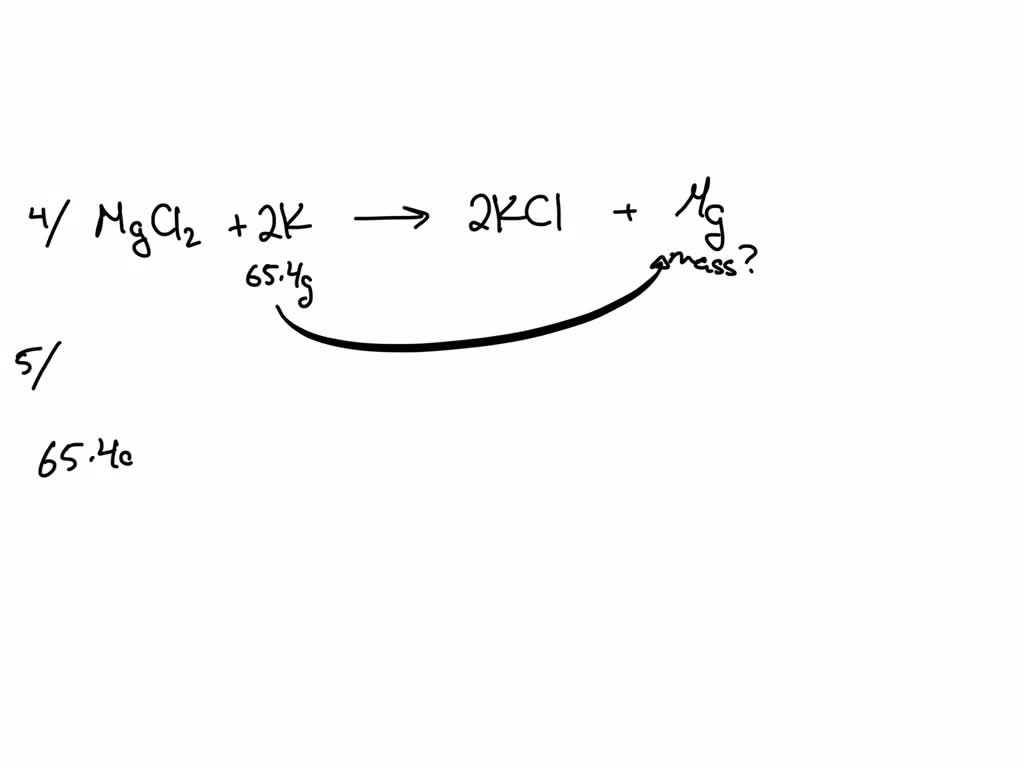
SOLVED Question 4 Magnesium chloride combines with potassium yielding
Magnesium + Zinc Chloride Equation Which of the following equations correctly. To balance a chemical equation,. The following equation describes a reaction between magnesium and zinc chloride: Hydrochloric acid + zinc → zinc chloride +. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). First, we set all coefficients to 1: Which of the following equations correctly. Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; Acid + metal → salt + hydrogen. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Let's balance this equation using the inspection method. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Magnesium + Zinc Chloride Equation Hydrochloric acid + zinc → zinc chloride +. The following equation describes a reaction between magnesium and zinc chloride: Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). Use the. Magnesium + Zinc Chloride Equation.
From www.chegg.com
A student wanted to illustrate the structure of Magnesium + Zinc Chloride Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). First, we set all coefficients to 1: 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Acid. Magnesium + Zinc Chloride Equation.
From gioxcpnsw.blob.core.windows.net
Magnesium And Zinc Sulfate Net Ionic Equation at Steve Williamson blog Magnesium + Zinc Chloride Equation 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn. Magnesium + Zinc Chloride Equation.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Magnesium + Zinc Chloride Equation Hydrochloric acid + zinc → zinc chloride +. For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). The following equation describes a reaction between magnesium and zinc chloride: Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Which of the following equations correctly. Mg + zncl2 = zn. Magnesium + Zinc Chloride Equation.
From www.youtube.com
How to draw dot and cross diagram of magnesium chloride ionic compound Magnesium + Zinc Chloride Equation The following equation describes a reaction between magnesium and zinc chloride: 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Which of the following equations. Magnesium + Zinc Chloride Equation.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium + Zinc Chloride Equation To balance a chemical equation,. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Use the calculator below to balance chemical equations and determine the type of reaction (instructions). First, we set all coefficients to 1: 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Mg + zncl2 = zn +. Magnesium + Zinc Chloride Equation.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Magnesium + Zinc Chloride Equation Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). To balance a chemical equation,. Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below;. Magnesium + Zinc Chloride Equation.
From www.showme.com
ShowMe magnesium chloride Magnesium + Zinc Chloride Equation Let's balance this equation using the inspection method. Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). To balance a chemical equation,. Hydrochloric acid + zinc → zinc chloride +. Which of the. Magnesium + Zinc Chloride Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Magnesium + Zinc Chloride Equation For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). To balance a chemical equation,. First, we set all coefficients to 1: Which of the following equations correctly. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Mg + zncl2 = zn. Magnesium + Zinc Chloride Equation.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Magnesium + Zinc Chloride Equation Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Acid + metal → salt + hydrogen. To balance a chemical equation,. Hydrochloric acid + zinc → zinc chloride +. Which of the following equations correctly. The following equation describes a reaction between magnesium and zinc chloride: Let's balance this equation using the inspection method. Acids will react with reactive. Magnesium + Zinc Chloride Equation.
From www.meritnation.com
Pl solve this 3 Complete the following word equations and write Magnesium + Zinc Chloride Equation For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). The following equation describes a reaction between magnesium and zinc chloride: Hydrochloric acid + zinc → zinc chloride +. Which of the following equations correctly. Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to. Magnesium + Zinc Chloride Equation.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Magnesium + Zinc Chloride Equation Acid + metal → salt + hydrogen. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). Mg + zncl2 =. Magnesium + Zinc Chloride Equation.
From www.numerade.com
SOLVED Question 4 Magnesium chloride combines with potassium yielding Magnesium + Zinc Chloride Equation Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). The following equation describes a reaction between magnesium and zinc chloride:. Magnesium + Zinc Chloride Equation.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Magnesium + Zinc Chloride Equation Acid + metal → salt + hydrogen. For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). Let's balance this equation using the inspection method. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Hydrochloric acid + zinc → zinc chloride +. Mg +. Magnesium + Zinc Chloride Equation.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation, free download ID5979486 Magnesium + Zinc Chloride Equation For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; Acid + metal → salt + hydrogen. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for. Magnesium + Zinc Chloride Equation.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium + Zinc Chloride Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Which of the following equations correctly. Acid + metal → salt + hydrogen. The following equation describes a reaction between magnesium and zinc chloride: Mg + zncl2 = zn + mgcl2 is a single displacement. Magnesium + Zinc Chloride Equation.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium + Zinc Chloride Equation To balance a chemical equation,. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Hydrochloric acid + zinc → zinc chloride +. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms. Magnesium + Zinc Chloride Equation.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium + Zinc Chloride Equation First, we set all coefficients to 1: Let's balance this equation using the inspection method. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Hydrochloric acid + zinc → zinc chloride +. Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; Which. Magnesium + Zinc Chloride Equation.
From ar.inspiredpencil.com
Magnesium Chloride Structure Magnesium + Zinc Chloride Equation 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. First, we set all coefficients to 1: Zinc metal reacts with aqueous hcl to give hydrogen. Magnesium + Zinc Chloride Equation.
From www.pw.live
Zinc Chloride Formula Magnesium + Zinc Chloride Equation Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). To balance a chemical equation,. Hydrochloric acid + zinc → zinc chloride +. Let's balance this equation using the inspection method. Which of the following equations correctly. Acid + metal → salt + hydrogen. The following equation describes a reaction between magnesium and zinc chloride: First, we set all coefficients. Magnesium + Zinc Chloride Equation.
From www.numerade.com
SOLVEDZinc and magnesium metal each reacts with hydrochloric acid to Magnesium + Zinc Chloride Equation Which of the following equations correctly. Acid + metal → salt + hydrogen. First, we set all coefficients to 1: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole. Magnesium + Zinc Chloride Equation.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Magnesium + Zinc Chloride Equation Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. First, we set all coefficients to 1: Acid + metal → salt + hydrogen. Hydrochloric acid + zinc → zinc chloride +. Which of the following equations correctly. Let's balance this equation using the. Magnesium + Zinc Chloride Equation.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Magnesium + Zinc Chloride Equation The following equation describes a reaction between magnesium and zinc chloride: To balance a chemical equation,. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Acid + metal → salt + hydrogen. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Zinc metal reacts with aqueous. Magnesium + Zinc Chloride Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Magnesium + Zinc Chloride Equation Hydrochloric acid + zinc → zinc chloride +. To balance a chemical equation,. Let's balance this equation using the inspection method. For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). The following equation describes a reaction between magnesium and zinc chloride: Use the calculator below to balance chemical equations. Magnesium + Zinc Chloride Equation.
From www.numerade.com
SOLVED Write the chemical equation for the reaction of magnesium with Magnesium + Zinc Chloride Equation Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a chemical equation,. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. For example, when magnesium (mg) reacts. Magnesium + Zinc Chloride Equation.
From www.toppr.com
Convert the following word equations into molecular equation and Magnesium + Zinc Chloride Equation 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. First, we set all coefficients to 1: Hydrochloric acid + zinc → zinc chloride +. Zinc metal. Magnesium + Zinc Chloride Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Magnesium + Zinc Chloride Equation Hydrochloric acid + zinc → zinc chloride +. Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. Which of the following equations correctly. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a chemical equation,.. Magnesium + Zinc Chloride Equation.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Magnesium + Zinc Chloride Equation Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. First, we set all coefficients to 1: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. To balance a chemical equation,. For example, when magnesium (mg). Magnesium + Zinc Chloride Equation.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium + Zinc Chloride Equation Which of the following equations correctly. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. First, we set all coefficients to 1: Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; To balance a chemical equation,. Zncl₂ (aq) + mg (s) mgcl₂. Magnesium + Zinc Chloride Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + Zn(NO3)2 = Mg(NO3)2 + Zn Magnesium + Zinc Chloride Equation Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Acid + metal → salt + hydrogen. 1 mg + 1 zncl 2 = 1 zn + 1 mgcl 2 for each element, we. For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). The following equation describes a reaction. Magnesium + Zinc Chloride Equation.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium + Zinc Chloride Equation The following equation describes a reaction between magnesium and zinc chloride: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Let's balance this equation using the inspection method. For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). 1 mg + 1. Magnesium + Zinc Chloride Equation.
From www.youtube.com
Write the chemical formula of the following compounds (a)Magnesium Magnesium + Zinc Chloride Equation For example, when magnesium (mg) reacts with copper (ii) sulfate (cuso 4), the magnesium atoms react with the copper (ii). First, we set all coefficients to 1: Hydrochloric acid + zinc → zinc chloride +. To balance a chemical equation,. Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown below; Zncl₂. Magnesium + Zinc Chloride Equation.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium + Zinc Chloride Equation Mg + zncl2 = zn + mgcl2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and one mole of aqueous zinc. To balance a chemical equation,. The following equation describes a reaction between magnesium and zinc chloride: Zinc metal reacts with aqueous hcl to give hydrogen gas and zinc chloride, according to the equation shown. Magnesium + Zinc Chloride Equation.
From www.toppr.com
Magnesium combines with chlorine to form magnesium chloride. The Magnesium + Zinc Chloride Equation Which of the following equations correctly. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. First, we set all coefficients to 1: Let's balance this equation using the inspection method. The following equation describes a reaction between magnesium and zinc chloride: Mg + zncl2 =. Magnesium + Zinc Chloride Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium + Zinc Chloride Equation The following equation describes a reaction between magnesium and zinc chloride: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zncl₂ (aq) + mg (s) mgcl₂ (aq) + zn (s). Which of the following equations correctly. To balance a chemical equation,. Acid + metal → salt + hydrogen. Zinc metal reacts with. Magnesium + Zinc Chloride Equation.