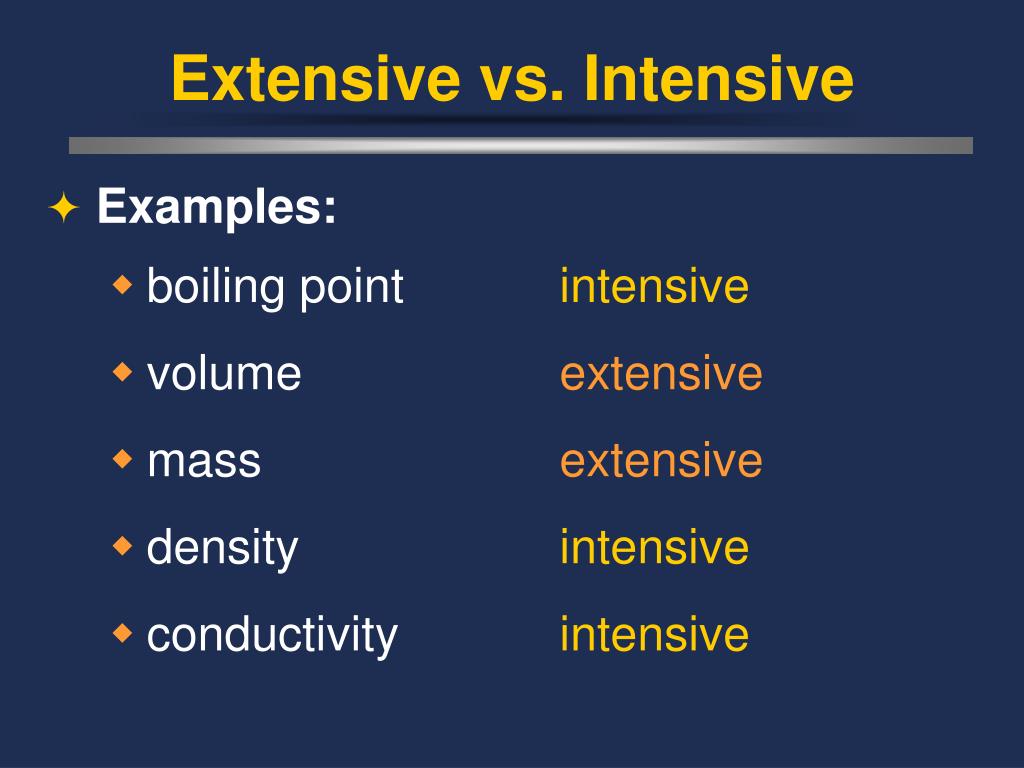
Is Phase Change Points Intensive Or Extensive . Examples include state of matter, temperature, and density. extensive properties vary with the amount of the substance and include mass, weight, and volume. The characteristics that distinguish one substance from another. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. intensive properties do not depend on the amount of matter in a substance. identify properties of matter as extensive or intensive. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system.
from www.slideserve.com
extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. extensive properties vary with the amount of the substance and include mass, weight, and volume. The characteristics that distinguish one substance from another. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. identify properties of matter as extensive or intensive. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. intensive properties do not depend on the amount of matter in a substance. Examples include state of matter, temperature, and density.
PPT Properties & Changes in Matter Extensive vs. Intensive Physical vs. Chemical PowerPoint
Is Phase Change Points Intensive Or Extensive intensive properties do not depend on the amount of matter in a substance. identify properties of matter as extensive or intensive. The characteristics that distinguish one substance from another. extensive properties vary with the amount of the substance and include mass, weight, and volume. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. intensive properties do not depend on the amount of matter in a substance. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Examples include state of matter, temperature, and density. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic.
From www.difference.minaprem.com
Difference Between Intensive Property and Extensive Property Is Phase Change Points Intensive Or Extensive Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Examples include state of matter, temperature, and density. The characteristics that distinguish one substance from another. intensive properties do not depend on. Is Phase Change Points Intensive Or Extensive.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Is Phase Change Points Intensive Or Extensive Examples include state of matter, temperature, and density. The characteristics that distinguish one substance from another. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. extensive properties vary with the amount of the substance and include mass, weight, and volume. intensive properties do not. Is Phase Change Points Intensive Or Extensive.
From sciencenotes.org
Difference Between Intensive and Extensive Properties of Matter Is Phase Change Points Intensive Or Extensive intensive properties do not depend on the amount of matter in a substance. identify properties of matter as extensive or intensive. extensive properties vary with the amount of the substance and include mass, weight, and volume. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Examples. Is Phase Change Points Intensive Or Extensive.
From pediaa.com
Difference Between Intensive and Extensive Properties Definition, Examples and Differences Is Phase Change Points Intensive Or Extensive extensive properties vary with the amount of the substance and include mass, weight, and volume. The characteristics that distinguish one substance from another. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. identify. Is Phase Change Points Intensive Or Extensive.
From general.chemistrysteps.com
Heat and Phase Change Diagrams Chemistry Steps Is Phase Change Points Intensive Or Extensive Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Examples include state of matter, temperature, and density. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. extensive properties vary with the amount of the substance and include mass, weight, and. Is Phase Change Points Intensive Or Extensive.
From www.101diagrams.com
Phase Change Diagrams 101 Diagrams Is Phase Change Points Intensive Or Extensive a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Examples include state of matter, temperature, and density. intensive properties do not depend on the amount of matter in a substance. identify properties of matter as extensive or intensive. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing,. Is Phase Change Points Intensive Or Extensive.
From brainly.ph
direction complete the concept map of phase change Brainly.ph Is Phase Change Points Intensive Or Extensive extensive properties vary with the amount of the substance and include mass, weight, and volume. Examples include state of matter, temperature, and density. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. The characteristics that distinguish one substance from another. a phase change or phase transition is a change between solid, liquid, gaseous,. Is Phase Change Points Intensive Or Extensive.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. extensive properties vary with the amount of the substance and include mass, weight, and volume. Examples include state of matter, temperature, and density. identify properties of matter as extensive or intensive. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on. Is Phase Change Points Intensive Or Extensive.
From www.seekpng.com
Types Of Phase Change Phase Transition (2364x1300), Png Download Is Phase Change Points Intensive Or Extensive extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Examples include state of matter, temperature, and density. The characteristics that distinguish one substance from another. extensive properties vary with the amount of the substance and include mass, weight, and volume. identify properties of matter. Is Phase Change Points Intensive Or Extensive.
From www.101diagrams.com
Phase Change Diagrams 101 Diagrams Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. intensive properties do not depend on the amount of matter in a substance. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Examples. Is Phase Change Points Intensive Or Extensive.
From www.slideserve.com
PPT Properties & Changes in Matter Extensive vs. Intensive Physical vs. Chemical PowerPoint Is Phase Change Points Intensive Or Extensive intensive properties do not depend on the amount of matter in a substance. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. The characteristics that distinguish one substance from another. Examples include state of. Is Phase Change Points Intensive Or Extensive.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Is Phase Change Points Intensive Or Extensive intensive properties do not depend on the amount of matter in a substance. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. extensive properties vary with the amount of the substance and include mass, weight, and volume. extensive properties depend on the amount of matter (extent of the system), while intensive do. Is Phase Change Points Intensive Or Extensive.
From www.thoughtco.com
List of Phase Changes Between States of Matter Is Phase Change Points Intensive Or Extensive extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Examples include state of matter, temperature, and density. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. extensive properties vary with the amount of the substance and include mass, weight, and. Is Phase Change Points Intensive Or Extensive.
From app.jove.com
Phase Diagram Concept Physics JoVe Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. identify properties of matter as extensive or intensive. intensive properties do not depend on the amount of matter in a substance. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. extensive properties depend on the amount of matter. Is Phase Change Points Intensive Or Extensive.
From unistudium.unipg.it
Phase Diagrams Is Phase Change Points Intensive Or Extensive intensive properties do not depend on the amount of matter in a substance. Examples include state of matter, temperature, and density. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. identify properties of matter as extensive or intensive. a phase change or phase. Is Phase Change Points Intensive Or Extensive.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3993444 Is Phase Change Points Intensive Or Extensive identify properties of matter as extensive or intensive. Examples include state of matter, temperature, and density. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. intensive properties do not depend. Is Phase Change Points Intensive Or Extensive.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Is Phase Change Points Intensive Or Extensive Examples include state of matter, temperature, and density. intensive properties do not depend on the amount of matter in a substance. The characteristics that distinguish one substance from another. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. identify properties of matter as extensive or intensive. . Is Phase Change Points Intensive Or Extensive.
From www.slideshare.net
Phase changes Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. Examples include state of matter, temperature, and density. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. extensive properties vary with the amount of the substance. Is Phase Change Points Intensive Or Extensive.
From www.britannica.com
Phase Definition & Facts Britannica Is Phase Change Points Intensive Or Extensive a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. Examples include state. Is Phase Change Points Intensive Or Extensive.
From www.slideserve.com
PPT Properties of Matter II PowerPoint Presentation, free download ID9698688 Is Phase Change Points Intensive Or Extensive intensive properties do not depend on the amount of matter in a substance. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. identify properties of matter as extensive or intensive.. Is Phase Change Points Intensive Or Extensive.
From www.sliderbase.com
Phase Diagrams Presentation Chemistry Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. identify properties of matter as extensive or intensive. extensive properties vary with the amount of the. Is Phase Change Points Intensive Or Extensive.
From allinonehighschool.com
Calculating Energy Change Phase Change Easy Peasy AllinOne High School Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. extensive properties vary with the amount of the substance and include mass, weight, and volume. Examples include state of matter, temperature, and density. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. identify properties of matter as extensive or. Is Phase Change Points Intensive Or Extensive.
From www.slideserve.com
PPT Phase Changes & Phase Diagrams PowerPoint Presentation, free download ID6240084 Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. identify properties of matter as extensive or intensive. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. a phase change or phase. Is Phase Change Points Intensive Or Extensive.
From nursehub.com
Phase Changes NurseHub Is Phase Change Points Intensive Or Extensive Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. intensive properties do not depend on the amount of matter in a substance. identify properties of matter as extensive or intensive. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. extensive properties. Is Phase Change Points Intensive Or Extensive.
From opentextbc.ca
Phase Changes Basic HVAC Is Phase Change Points Intensive Or Extensive extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. intensive properties do not depend on the amount of matter in a substance. identify properties of matter as extensive or intensive. extensive properties vary with the amount of the substance and include mass, weight,. Is Phase Change Points Intensive Or Extensive.
From www.thoughtco.com
The Difference Between Intensive and Extensive Properties Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. identify properties of matter as extensive or intensive. Examples include state of matter, temperature, and density. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. extensive properties depend on the amount of matter (extent of the system), while intensive. Is Phase Change Points Intensive Or Extensive.
From www.expii.com
Extensive vs. Intensive Properties — Overview & Examples Expii Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. identify properties of matter as extensive or intensive. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Examples include state of matter, temperature, and density. intensive properties do not depend on the amount of matter in a substance. . Is Phase Change Points Intensive Or Extensive.
From wiringdiagramidealless.z21.web.core.windows.net
How To Read A Phase Change Diagram Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. extensive properties vary with the amount of the substance and include mass, weight, and volume. intensive properties do not depend on the amount of matter in a substance. identify properties of matter as extensive or. Is Phase Change Points Intensive Or Extensive.
From www.slideshare.net
Phase changes Is Phase Change Points Intensive Or Extensive a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. The characteristics that distinguish one substance from another. Examples include state of matter, temperature, and density. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. . Is Phase Change Points Intensive Or Extensive.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Is Phase Change Points Intensive Or Extensive a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Examples include state of matter, temperature, and density. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing,. Is Phase Change Points Intensive Or Extensive.
From www.jove.com
Phase Diagrams Carbon Dioxide and Water Phase Diagrams Chemistry JoVE Is Phase Change Points Intensive Or Extensive a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. identify properties of matter as extensive or intensive. extensive properties depend on the amount of matter (extent of the system), while intensive do not. Is Phase Change Points Intensive Or Extensive.
From dokumen.tips
(PPTX) Lesson 5 CHANGE OF PHASE DISTINGUISH between intensive and extensive properties. DEFINE Is Phase Change Points Intensive Or Extensive intensive properties do not depend on the amount of matter in a substance. The characteristics that distinguish one substance from another. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. identify properties of matter as extensive or intensive. extensive properties vary with the. Is Phase Change Points Intensive Or Extensive.
From chem.libretexts.org
10.5 Phase Transitions Chemistry LibreTexts Is Phase Change Points Intensive Or Extensive extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. a phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. extensive properties. Is Phase Change Points Intensive Or Extensive.
From www.youtube.com
Phase Change Diagrams YouTube Is Phase Change Points Intensive Or Extensive extensive properties vary with the amount of the substance and include mass, weight, and volume. intensive properties do not depend on the amount of matter in a substance. Examples include state of matter, temperature, and density. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of. Is Phase Change Points Intensive Or Extensive.
From kentchemistry.com
Phase Changes Is Phase Change Points Intensive Or Extensive The characteristics that distinguish one substance from another. extensive properties depend on the amount of matter (extent of the system), while intensive do not depend on the amount of system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic. intensive properties do not depend on the amount of matter in a substance. . Is Phase Change Points Intensive Or Extensive.