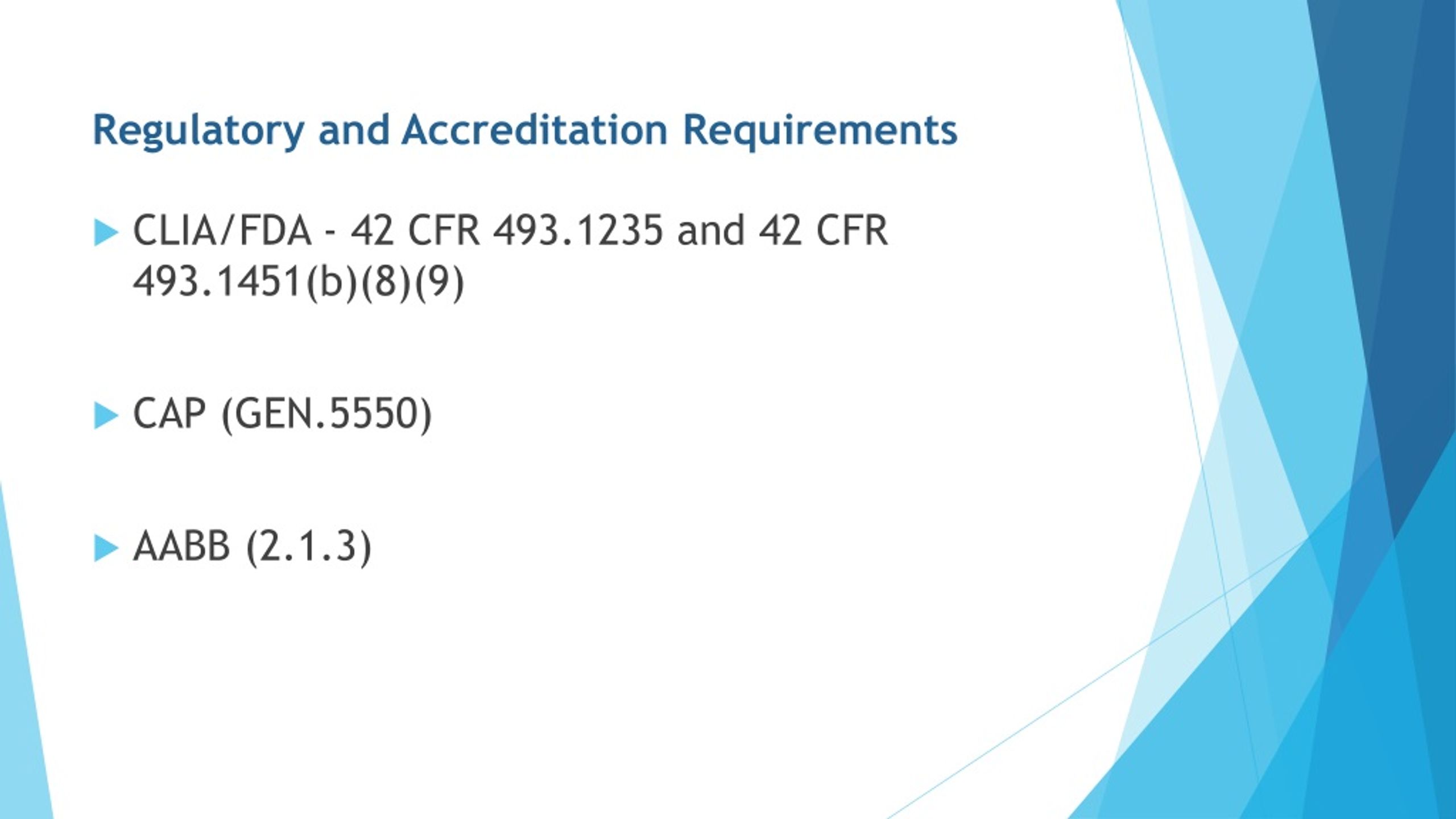
Lab Manager Requirements Clia . Laboratories must have one or more individuals who can meet the. a clia lab director meets clia requirements regarding experience, education, and training. in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. They’re also responsible for the lab. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). the top ten tips you should consider as a laboratory director are: the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. clia clinical laboratory personnel requirements.
from www.slideserve.com
the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. clia clinical laboratory personnel requirements. the top ten tips you should consider as a laboratory director are: the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. a clia lab director meets clia requirements regarding experience, education, and training. in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. They’re also responsible for the lab. Laboratories must have one or more individuals who can meet the.
PPT Complying With CLIA Competency Requirements PowerPoint Presentation ID8999050
Lab Manager Requirements Clia the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). a clia lab director meets clia requirements regarding experience, education, and training. the top ten tips you should consider as a laboratory director are: the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. clia clinical laboratory personnel requirements. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). They’re also responsible for the lab. in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. Laboratories must have one or more individuals who can meet the.
From www.slideserve.com
PPT Complying With CLIA Competency Requirements PowerPoint Presentation ID8999050 Lab Manager Requirements Clia Laboratories must have one or more individuals who can meet the. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. a clia lab director meets clia requirements regarding experience, education, and training. They’re also responsible for. Lab Manager Requirements Clia.
From www.labmanager.com
CLIA Compliance for PreAnalytic, Analytic, and PostAnalytic Testing Phases Lab Manager Lab Manager Requirements Clia the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. They’re also responsible for the lab. in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. Laboratories must have one or more individuals who can meet the. . Lab Manager Requirements Clia.
From www.collaborativetherapeutics.com
Ultimate CLIA Lab Building Checklist Lab Manager Requirements Clia the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. a clia lab director meets clia requirements regarding experience, education, and training. They’re also responsible for the lab. in those instances where all of the laboratories. Lab Manager Requirements Clia.
From www.slideserve.com
PPT LABORATORY MANAGEMENT REVIEW 2008 PowerPoint Presentation, free download ID4996147 Lab Manager Requirements Clia clia clinical laboratory personnel requirements. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). Laboratories must have one or more individuals who can meet the. They’re also responsible for the lab. the top ten tips you should consider as a laboratory director are: the laboratory director must be qualified to manage and. Lab Manager Requirements Clia.
From www.medialab.com
Compass for POCT & PPMP Enforce CLIA Competency Assessment Procedures Lab Manager Requirements Clia in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. clia clinical laboratory personnel requirements. They’re also responsible for the lab. a clia lab director meets clia requirements regarding experience, education, and training. the top ten tips you should consider as a laboratory director. Lab Manager Requirements Clia.
From www.mlo-online.com
CLIA and regulatory readiness How can your lab always be ready? Lab Manager Requirements Clia the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). a clia lab director meets clia requirements regarding experience, education, and training. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. clia clinical laboratory personnel requirements. the top ten tips you should consider. Lab Manager Requirements Clia.
From www.laboratoryeconomics.com
The CLIA Compliance Reference Guide for Laboratory Managers Lab Manager Requirements Clia a clia lab director meets clia requirements regarding experience, education, and training. Laboratories must have one or more individuals who can meet the. clia clinical laboratory personnel requirements. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. the top ten tips you should consider as a laboratory. Lab Manager Requirements Clia.
From www.thermofisher.com
Working with CLIA Lab Regulations LIMS System Lab Manager Requirements Clia clia clinical laboratory personnel requirements. Laboratories must have one or more individuals who can meet the. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. They’re also responsible for the lab. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. a clia lab director. Lab Manager Requirements Clia.
From www.scribd.com
CLIA Regulations Pertaining To Your Lab Quality Assurance Food And Drug Administration Lab Manager Requirements Clia a clia lab director meets clia requirements regarding experience, education, and training. the top ten tips you should consider as a laboratory director are: the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval. Lab Manager Requirements Clia.
From www.lighthouselabservices.com
What Is a CLIA Lab Director and What Are Their Requirements? Lab Manager Requirements Clia the top ten tips you should consider as a laboratory director are: the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. Laboratories must have one or more individuals who can meet the. clia clinical laboratory personnel requirements. in those instances where all of the laboratories in a. Lab Manager Requirements Clia.
From www.youtube.com
2020 04 14 ACIL Clinical Laboratory Improvement Amendments CLIA Certification Process inar Lab Manager Requirements Clia Laboratories must have one or more individuals who can meet the. the top ten tips you should consider as a laboratory director are: the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. They’re also responsible for the lab. in those instances where all of the laboratories in a. Lab Manager Requirements Clia.
From www.slideserve.com
PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1222846 Lab Manager Requirements Clia the top ten tips you should consider as a laboratory director are: the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). clia clinical laboratory personnel requirements. Laboratories must have one or more individuals who can meet the. a clia. Lab Manager Requirements Clia.
From www.scribd.com
Clia Lab Qualifications Doctor Of Medicine Medical Laboratory Lab Manager Requirements Clia the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. a clia lab director meets clia requirements regarding experience, education, and training. They’re also responsible for the lab. clia clinical laboratory. Lab Manager Requirements Clia.
From www.laboratoryeconomics.com
CLIA DATABASE of 33,000 LABS LABORATORY ECONOMiCS Lab Manager Requirements Clia the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. clia clinical laboratory personnel requirements. the top ten tips you should consider as a laboratory director are: Laboratories must have one. Lab Manager Requirements Clia.
From www.youtube.com
CLIA Certificate of Waiver Navigating Medical Laboratory Requirements YouTube Lab Manager Requirements Clia the top ten tips you should consider as a laboratory director are: the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. clia clinical laboratory personnel requirements. They’re also responsible for the lab. the laboratory director must be qualified to. Lab Manager Requirements Clia.
From www.collaborativetherapeutics.com
Ultimate CLIA Lab Building Checklist Lab Manager Requirements Clia the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the top ten tips you should consider as a laboratory director are: . Lab Manager Requirements Clia.
From www.slideserve.com
PPT CallIn Information PowerPoint Presentation, free download ID4735038 Lab Manager Requirements Clia Laboratories must have one or more individuals who can meet the. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. the top ten tips you should consider as a laboratory director are: in those instances where all of the laboratories in a state are exempt from clia requirements,. Lab Manager Requirements Clia.
From www.researchgate.net
Overview of Clinical Laboratory Improvement Amendments (CLIA) model,... Download Table Lab Manager Requirements Clia in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. clia clinical laboratory personnel requirements. They’re also responsible for the lab. the centers for medicare & medicaid services (cms) clinical laboratory improvement. Lab Manager Requirements Clia.
From studylib.net
Lab Regulations MI CLIA Policy Lab Manager Requirements Clia They’re also responsible for the lab. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). clia clinical laboratory personnel requirements. in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. a clia lab director meets clia requirements regarding experience, education,. Lab Manager Requirements Clia.
From go.labmanager.com
Standard Operating Procedures for CLIA Compliance Lab Manager Requirements Clia They’re also responsible for the lab. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. clia clinical laboratory personnel requirements. the top ten tips you should consider as a laboratory director are: in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of. Lab Manager Requirements Clia.
From www.scribd.com
CLIA Requirements For Proficiency Testing The Basics For Laboratory Professionals PDF Lab Manager Requirements Clia the top ten tips you should consider as a laboratory director are: in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. Laboratories must have one or more individuals who can meet the. They’re also responsible for the lab. the centers for medicare & medicaid. Lab Manager Requirements Clia.
From www.slideserve.com
PPT LRI Validation Suite Meeting PowerPoint Presentation, free download ID6507574 Lab Manager Requirements Clia clia clinical laboratory personnel requirements. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). They’re also responsible for the lab. the top ten tips you should consider as a laboratory director are: the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. in. Lab Manager Requirements Clia.
From www.slideserve.com
PPT LABORATORY MANAGEMENT REVIEW 2008 PowerPoint Presentation, free download ID4996147 Lab Manager Requirements Clia the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. They’re also responsible for the lab. the top ten tips you should consider as a laboratory director are: Laboratories must have one or more individuals who can meet the. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). clia clinical. Lab Manager Requirements Clia.
From www.slideserve.com
PPT QUALITY ASSURANCE PowerPoint Presentation, free download ID1409864 Lab Manager Requirements Clia the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. a clia lab director meets clia requirements regarding experience, education, and training. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). the top ten tips you should consider as a laboratory director are: Laboratories must have one or more individuals. Lab Manager Requirements Clia.
From www.slideserve.com
PPT Complying With CLIA Competency Requirements PowerPoint Presentation ID8999050 Lab Manager Requirements Clia the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). clia clinical laboratory personnel requirements. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. They’re also responsible for the lab. Laboratories must have one or more individuals who can meet the. the top ten. Lab Manager Requirements Clia.
From exodvatfl.blob.core.windows.net
Clia Requirements Lab Director at Ronald Roche blog Lab Manager Requirements Clia the top ten tips you should consider as a laboratory director are: in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the centers for medicare & medicaid services (cms) clinical laboratory. Lab Manager Requirements Clia.
From www.g2intelligence.com
An Analysis of CMS’s Proposed Changes to CLIA Lab Personnel Requirements Lab Manager Requirements Clia the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). They’re also responsible for the lab. in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. the top ten. Lab Manager Requirements Clia.
From iqlabconsultants.com
Ensuring Compliance In Your Lab CLIA Regulations Demystified Lab Consulting Services Lab Manager Requirements Clia the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). Laboratories must have one or more individuals who can meet the. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the top. Lab Manager Requirements Clia.
From www.slideshare.net
Setting Up a CLIA Lab Lab Manager Requirements Clia the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). a clia lab director meets clia requirements regarding experience, education, and training. the top ten tips you should consider as a laboratory director are: the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. They’re. Lab Manager Requirements Clia.
From studylib.net
Assessment of Lab Personnel Performing CLIA Approved Lab Manager Requirements Clia the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. Laboratories must have one or more individuals who can meet the. They’re also responsible for the lab. clia clinical laboratory personnel requirements. in those instances where all of the laboratories in a state are exempt from clia requirements, based. Lab Manager Requirements Clia.
From hometestbox.com
What Is a CLIACertified Lab? Home Test Box Lab Manager Requirements Clia a clia lab director meets clia requirements regarding experience, education, and training. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. the top ten tips you should consider as a. Lab Manager Requirements Clia.
From www.stuvia.com
CLIA Regulations and Lab management questions and answers 2024 with complete solution CLIA Lab Manager Requirements Clia a clia lab director meets clia requirements regarding experience, education, and training. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). Laboratories must have one or more individuals who can meet the. in those. Lab Manager Requirements Clia.
From vdocuments.mx
CLIA Clinical Laboratory Personnel Requirements … Clinical Laboratory Personnel Requirements Lab Manager Requirements Clia the top ten tips you should consider as a laboratory director are: in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. a clia lab director meets clia requirements regarding experience, education,. Lab Manager Requirements Clia.
From www.mybiosource.com
Ensuring Quality and Accuracy in Laboratory Testing An Overview of CLIA Regulations Lab Manager Requirements Clia the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. They’re also responsible for the lab. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. Laboratories must have one or more individuals who can meet the. the centers for medicare & medicaid services (cms) clinical laboratory. Lab Manager Requirements Clia.
From www.slideserve.com
PPT CallIn Information PowerPoint Presentation, free download ID4735038 Lab Manager Requirements Clia in those instances where all of the laboratories in a state are exempt from clia requirements, based on the approval of a. the laboratory director must be qualified to manage and direct the laboratory personnel and the performance of moderate. the centers for medicare & medicaid services (cms) clinical laboratory improvement amendments (clia). the clinical laboratory. Lab Manager Requirements Clia.