What Is The Purpose Of Ice-Cold Ethanol Addition . when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. The cold water protects the dna by slowing down enzymes that can break it. Dna is soluble in water but insoluble in the presence.
from www.chegg.com
when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. The cold water protects the dna by slowing down enzymes that can break it. Dna is soluble in water but insoluble in the presence. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular.
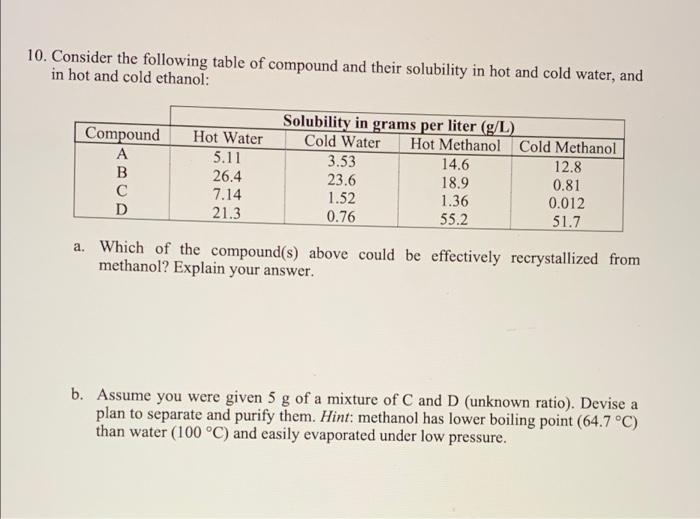
Solved 10. Consider the following table of compound and
What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is soluble in water but insoluble in the presence. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. The cold water protects the dna by slowing down enzymes that can break it.
From socratic.org
How can alkenes be used to make ethanol? Socratic What Is The Purpose Of Ice-Cold Ethanol Addition if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is soluble in water but insoluble in the presence. The cold water protects the dna by slowing down enzymes that can break it. when cold ethanol is added to the filtrate, dna precipitates at. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
Impact of ethanol addition on vapor pressure change (ΔVP) of commercial... Download Scientific What Is The Purpose Of Ice-Cold Ethanol Addition if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is soluble in water but insoluble in the presence. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. The cold water protects the dna by slowing down enzymes that. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.blog44.ca
Strawberry Lab and DNA Model Claire Teegen What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble in the presence. The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.chegg.com
Solved 9. It is desired to heat cold ethanol in a What Is The Purpose Of Ice-Cold Ethanol Addition if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is soluble in water but insoluble in the presence. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle. What Is The Purpose Of Ice-Cold Ethanol Addition.
From exoqxqoqd.blob.core.windows.net
Why Is The Cold Ethanol Added To The Soap And Salt Mixture at Jon Flowers blog What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. Dna is soluble in water but insoluble in the presence. if you are precipitating small volumes of dna, and you can fit the required. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.slideserve.com
PPT Relevance of DNA Isolation PowerPoint Presentation, free download ID4748560 What Is The Purpose Of Ice-Cold Ethanol Addition The cold water protects the dna by slowing down enzymes that can break it. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.slideserve.com
PPT DNA Extraction PowerPoint Presentation, free download ID1925645 What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. finally, ethanol is added causing the. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
Effect of ethanol addition at various time intervals. Oil and urea... Download Scientific Diagram What Is The Purpose Of Ice-Cold Ethanol Addition The cold water protects the dna by slowing down enzymes that can break it. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. if you are precipitating small volumes of dna, and you. What Is The Purpose Of Ice-Cold Ethanol Addition.
From studylib.net
The effect of ethanol on melting ice What Is The Purpose Of Ice-Cold Ethanol Addition Dna is soluble in water but insoluble in the presence. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
Effect of ethanol addition on amount of LTHR. Download Scientific Diagram What Is The Purpose Of Ice-Cold Ethanol Addition The cold water protects the dna by slowing down enzymes that can break it. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. Dna is soluble in water but insoluble in the presence. . What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.numerade.com
SOLVED 1. Salt is added to sample for DNA extraction for two reasons. What are both of the What Is The Purpose Of Ice-Cold Ethanol Addition The cold water protects the dna by slowing down enzymes that can break it. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is soluble in water but insoluble. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.slideserve.com
PPT DNA Extraction from Cheek Cells PowerPoint Presentation, free download ID2327842 What Is The Purpose Of Ice-Cold Ethanol Addition Dna is soluble in water but insoluble in the presence. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. The cold water protects the dna by slowing down enzymes that can break it. finally, ethanol is added causing the dna to precipitate (settle out). What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.chegg.com
Solved CH3Br ethanol, cold What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. The cold water protects the dna by slowing down enzymes that can break it. when. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
Contents of total polyphenols and flavonoids in water and ethanol... Download Scientific Diagram What Is The Purpose Of Ice-Cold Ethanol Addition Dna is soluble in water but insoluble in the presence. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. The cold water protects the dna by slowing down enzymes that can break it. . What Is The Purpose Of Ice-Cold Ethanol Addition.
From slideplayer.com
Lecture 5c Aldol Condensation. Introduction The acidity of organic compounds is often determined What Is The Purpose Of Ice-Cold Ethanol Addition if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. The cold water protects the dna by slowing down enzymes that can break it. finally, ethanol is added causing the. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.coursehero.com
[Solved] A student testing the solubility of NaCl in icecold ethanol... Course Hero What Is The Purpose Of Ice-Cold Ethanol Addition The cold water protects the dna by slowing down enzymes that can break it. Dna is soluble in water but insoluble in the presence. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. finally, ethanol is added causing the dna to precipitate (settle out). What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.youtube.com
Ethanol Precipitaion of DNA YouTube What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. when. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
a,b) Photographs of a pure ice immersed in ethanol (−20 °C) and ethyl... Download Scientific What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble in the presence. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. finally, ethanol is added causing the dna to precipitate (settle. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
View of the addition of cold ethanol to the zinc liquid Download Scientific Diagram What Is The Purpose Of Ice-Cold Ethanol Addition The cold water protects the dna by slowing down enzymes that can break it. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is soluble in water but insoluble. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.teachoo.com
[Chemistry] Differentiate between Ethanol and Ethanoic acid Class 10 What Is The Purpose Of Ice-Cold Ethanol Addition if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. Dna is soluble in water but insoluble in the presence. when cold ethanol is added. What Is The Purpose Of Ice-Cold Ethanol Addition.
From worksheetfullclement.z13.web.core.windows.net
Extracting Dna From Strawberries What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble in the presence. The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.numerade.com
SOLVED During this lab, the following balanced reaction will be done to make aspirin OH OH OH What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. The cold water protects the dna by slowing down enzymes that can break it. Dna is soluble in water but insoluble in the presence. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample. What Is The Purpose Of Ice-Cold Ethanol Addition.
From slideplayer.com
Extracting DNA from a strawberry ppt download What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. Dna is. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.organicchemistrytutor.com
Reactions of Alcohols — Organic Chemistry Tutor What Is The Purpose Of Ice-Cold Ethanol Addition Dna is soluble in water but insoluble in the presence. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. The cold water protects the dna by slowing down enzymes that can break it. . What Is The Purpose Of Ice-Cold Ethanol Addition.
From saylordotorg.github.io
Properties of Liquids What Is The Purpose Of Ice-Cold Ethanol Addition Dna is soluble in water but insoluble in the presence. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. if you are precipitating small volumes of dna, and you can fit the required. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.chegg.com
Solved 10. Consider the following table of compound and What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. Dna is soluble in water but insoluble in the presence. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. The cold water protects the dna by slowing down enzymes that can break it. . What Is The Purpose Of Ice-Cold Ethanol Addition.
From mavink.com
Ethanol Water Freezing Point Chart What Is The Purpose Of Ice-Cold Ethanol Addition Dna is soluble in water but insoluble in the presence. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. if you are precipitating small volumes of dna, and you can fit the required. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.slideserve.com
PPT Molecular Biology (MLMB201) PowerPoint Presentation, free download ID5948992 What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble in the presence. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. The cold water protects the dna by slowing down enzymes that. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.youtube.com
Dry ice placed in glycerol, ethanol, and water. YouTube What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble in the presence. The cold water protects the dna by slowing down enzymes that can break it. . What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
Schematic illustration of the strategies of generating a liquid layer... Download Scientific What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble in the presence. The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
View of the addition of cold ethanol to the zinc liquid Download Scientific Diagram What Is The Purpose Of Ice-Cold Ethanol Addition when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble in the presence. The cold water protects the dna by slowing down enzymes that can break it. finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. . What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.slideserve.com
PPT DNA Extraction from Cheek Cells PowerPoint Presentation, free download ID2327842 What Is The Purpose Of Ice-Cold Ethanol Addition Dna is soluble in water but insoluble in the presence. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. The cold water protects the dna by slowing down enzymes that. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.researchgate.net
(PDF) Ethanol, fuel for CO2 reduction on ICE What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. Dna is soluble in water but insoluble in the presence. The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount. What Is The Purpose Of Ice-Cold Ethanol Addition.
From sciencenote.info
What is the freezing point of ethanol? ScienceNote.info What Is The Purpose Of Ice-Cold Ethanol Addition finally, ethanol is added causing the dna to precipitate (settle out) of the solution, leaving behind all the cellular. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. The cold water protects the dna by slowing down enzymes that can break it. Dna is. What Is The Purpose Of Ice-Cold Ethanol Addition.
From www.chegg.com
Solved Determine the limiting reagent in the synthesis of What Is The Purpose Of Ice-Cold Ethanol Addition The cold water protects the dna by slowing down enzymes that can break it. if you are precipitating small volumes of dna, and you can fit the required amount of solvent into the sample tube, then ice. when cold ethanol is added to the filtrate, dna precipitates at the water/ethanol interface. Dna is soluble in water but insoluble. What Is The Purpose Of Ice-Cold Ethanol Addition.