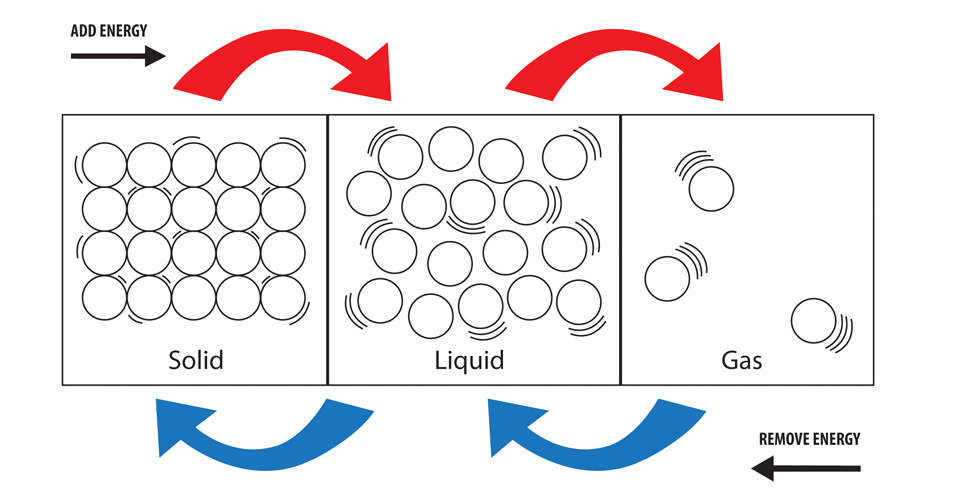
Solid To Liquid Heat Energy . Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The energy required by different. The amount of energy required to sublime 1 mol of a pure solid is. The atoms of a solid are held together by chemical bonds. Changes in a material's temperature or state of matter are caused by changes to the internal energy. When a solid like my ice lolly is heated, it melts to become a liquid. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. When part of a solid absorbs. The atoms are fixed in place but are free to vibrate. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. And if you heat up a liquid even more, it’ll evaporate to become a gas, also.
from socratic.org
When a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The atoms of a solid are held together by chemical bonds. The atoms are fixed in place but are free to vibrate. When part of a solid absorbs. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The energy required by different.
What happens to the molecules in matter when you raise the temperature
Solid To Liquid Heat Energy And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The atoms of a solid are held together by chemical bonds. The energy required by different. When part of a solid absorbs. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. When a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The amount of energy required to sublime 1 mol of a pure solid is. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The atoms are fixed in place but are free to vibrate.
From www.sciencelearn.org.nz
Heat and change of state — Science Learning Hub Solid To Liquid Heat Energy When part of a solid absorbs. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. When a solid like my ice lolly is heated, it melts to become a liquid. The amount of energy required to sublime 1 mol of a pure solid is. If energy is supplied by heating a solid,. Solid To Liquid Heat Energy.
From www.slideserve.com
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint Solid To Liquid Heat Energy The amount of energy required to sublime 1 mol of a pure solid is. The energy required by different. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The atoms of a solid are held together by chemical bonds. The atoms are fixed in place but are free to vibrate. Changes in. Solid To Liquid Heat Energy.
From taylorsciencegeeks.weebly.com
Methods of Heat Transfer Conduction Science News Solid To Liquid Heat Energy Changes in a material's temperature or state of matter are caused by changes to the internal energy. When a solid like my ice lolly is heated, it melts to become a liquid. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The atoms of a solid are held together by chemical bonds.. Solid To Liquid Heat Energy.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Heat Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The atoms of a solid are held together by chemical bonds. The energy required by different. Changes in a material's temperature or state of matter are caused. Solid To Liquid Heat Energy.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Heat Energy The atoms are fixed in place but are free to vibrate. Changes in a material's temperature or state of matter are caused by changes to the internal energy. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Energy. Solid To Liquid Heat Energy.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid To Liquid Heat Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. When a solid like my ice lolly is heated, it melts to become a liquid. The atoms are fixed in place but are free to vibrate. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The. Solid To Liquid Heat Energy.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid To Liquid Heat Energy Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. When part of a solid absorbs. The atoms of a solid are held together by chemical bonds. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the.. Solid To Liquid Heat Energy.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to Solid To Liquid Heat Energy Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The energy required by different. The atoms of a solid are held together by chemical bonds. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have. Solid To Liquid Heat Energy.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid To Liquid Heat Energy The energy required by different. The amount of energy required to sublime 1 mol of a pure solid is. The atoms of a solid are held together by chemical bonds. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Energy is required to melt a solid because the cohesive bonds between the. Solid To Liquid Heat Energy.
From www.slideserve.com
PPT Temperature, Heat, and Thermal Energy PowerPoint Presentation Solid To Liquid Heat Energy And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. When a solid like my ice lolly is heated, it melts to become a liquid. The atoms are fixed in place but are free to vibrate. Changes. Solid To Liquid Heat Energy.
From www.youtube.com
Physics Energy Heat Transfer Solids Liquids and Gases YouTube Solid To Liquid Heat Energy The energy required by different. The atoms of a solid are held together by chemical bonds. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. When part of. Solid To Liquid Heat Energy.
From gcsephysicsninja.com
3. Energy of solids, liquids and gases Solid To Liquid Heat Energy When a solid like my ice lolly is heated, it melts to become a liquid. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of energy required to sublime 1 mol of a pure solid is. The atoms of a solid are held together by chemical bonds. The atoms are. Solid To Liquid Heat Energy.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Solid To Liquid Heat Energy The amount of energy required to sublime 1 mol of a pure solid is. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The atoms are fixed in place but are free to vibrate. When part of a solid absorbs. When a solid like my ice lolly is heated, it melts to. Solid To Liquid Heat Energy.
From socratic.org
What happens to the molecules in matter when you raise the temperature Solid To Liquid Heat Energy Changes in a material's temperature or state of matter are caused by changes to the internal energy. The energy required by different. The atoms are fixed in place but are free to vibrate. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The atoms of a solid are held together by chemical bonds.. Solid To Liquid Heat Energy.
From www.scribd.com
The Effect of Heat On Matter PDF Liquids Solid Solid To Liquid Heat Energy When a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. When part of a solid absorbs. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. If energy is supplied by heating. Solid To Liquid Heat Energy.
From mungfali.com
Solid Liquid Gas Anchor Chart Solid To Liquid Heat Energy And if you heat up a liquid even more, it’ll evaporate to become a gas, also. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Changes in a material's temperature or state of matter are caused by changes. Solid To Liquid Heat Energy.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Solid To Liquid Heat Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The atoms of a solid are held together by chemical bonds. The atoms are fixed in place but are free to vibrate. The energy required. Solid To Liquid Heat Energy.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Solid To Liquid Heat Energy Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. When a solid like my ice lolly is heated, it melts to become a liquid. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The atoms. Solid To Liquid Heat Energy.
From www.slideserve.com
PPT Solids, Liquids, Energy & Heat PowerPoint Presentation, free Solid To Liquid Heat Energy And if you heat up a liquid even more, it’ll evaporate to become a gas, also. When a solid like my ice lolly is heated, it melts to become a liquid. The energy required by different. The atoms of a solid are held together by chemical bonds. When part of a solid absorbs. The direct conversion of a solid to. Solid To Liquid Heat Energy.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet Solid To Liquid Heat Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. When part of a solid absorbs. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. When a solid like my ice lolly is heated, it melts to become a liquid. Changes in a material's temperature or. Solid To Liquid Heat Energy.
From easyscienceforkids.com
Facts About States of Matter Easy Science For KidsEasy Science For Kids Solid To Liquid Heat Energy When part of a solid absorbs. The atoms of a solid are held together by chemical bonds. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Energy is required to melt a solid because. Solid To Liquid Heat Energy.
From www.vrogue.co
Convection Currents Vector Illustration Labeled Diagr vrogue.co Solid To Liquid Heat Energy If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. The atoms are fixed in place but are free to vibrate. The atoms of a solid are held together by chemical bonds. When part of a solid absorbs. And. Solid To Liquid Heat Energy.
From www.slideserve.com
PPT Chapter 16 Thermal properties of matter PowerPoint Presentation Solid To Liquid Heat Energy Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The atoms are fixed in place but are free to vibrate. If energy is supplied by heating a. Solid To Liquid Heat Energy.
From bleckinger9sci.weebly.com
2. Chemistry Bleckinger Year 9 Science Solid To Liquid Heat Energy The atoms are fixed in place but are free to vibrate. The energy required by different. When a solid like my ice lolly is heated, it melts to become a liquid. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The amount of energy. Solid To Liquid Heat Energy.
From www.slideshare.net
Chapter 24 Conduction Solid To Liquid Heat Energy The atoms are fixed in place but are free to vibrate. The energy required by different. When a solid like my ice lolly is heated, it melts to become a liquid. The amount of energy required to sublime 1 mol of a pure solid is. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until. Solid To Liquid Heat Energy.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid To Liquid Heat Energy When a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The atoms are fixed in place but are free to vibrate. The energy required by different. The direct conversion of a solid to a gas, without an intervening liquid. Solid To Liquid Heat Energy.
From stock.adobe.com
Thermal expansion of solids and liquids. The tendency of materials to Solid To Liquid Heat Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. When part of a solid absorbs. Energy is required to melt a. Solid To Liquid Heat Energy.
From www.pinterest.com
Latent Heat the heat energy that must be absorbed when a substance Solid To Liquid Heat Energy The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of energy required to sublime 1 mol of a pure solid is. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The atoms are fixed. Solid To Liquid Heat Energy.
From stock.adobe.com
State of matter infographic diagram solid liquid gas plasma heating Solid To Liquid Heat Energy Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. And if you heat up a liquid even more, it’ll evaporate to become a gas, also. The atoms of a solid are held together by chemical bonds. The amount of energy required to sublime 1. Solid To Liquid Heat Energy.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Heat Energy When a solid like my ice lolly is heated, it melts to become a liquid. The energy required by different. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The amount of energy required to sublime 1 mol of a pure solid is. Energy is required to melt a solid because the. Solid To Liquid Heat Energy.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid To Liquid Heat Energy Changes in a material's temperature or state of matter are caused by changes to the internal energy. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the. Solid To Liquid Heat Energy.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Solid To Liquid Heat Energy Changes in a material's temperature or state of matter are caused by changes to the internal energy. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The atoms are fixed in place but are free to vibrate. The direct conversion of a solid to a gas, without an intervening liquid phase,. Solid To Liquid Heat Energy.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Solid To Liquid Heat Energy When a solid like my ice lolly is heated, it melts to become a liquid. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. The atoms of a solid are held together by chemical bonds. The atoms are. Solid To Liquid Heat Energy.
From www.britannica.com
phase Definition & Facts Britannica Solid To Liquid Heat Energy When part of a solid absorbs. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The energy required by different. The atoms of a solid are held together by chemical bonds. When a solid like. Solid To Liquid Heat Energy.
From www.slideserve.com
PPT CHAPTER 7 HEAT PowerPoint Presentation, free download ID2090526 Solid To Liquid Heat Energy When a solid like my ice lolly is heated, it melts to become a liquid. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The atoms are fixed in place but are free to vibrate. When part of a solid absorbs. And if you heat up a liquid even more, it’ll. Solid To Liquid Heat Energy.