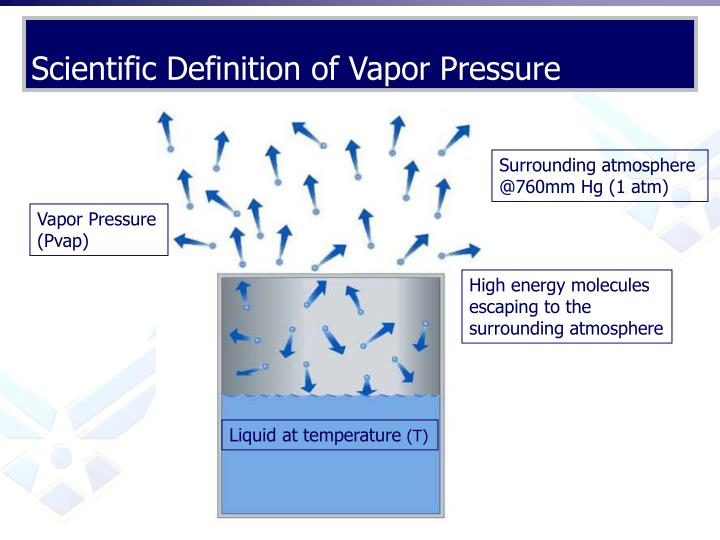
Vapor Definition Engineering . Vapor and saturation pressure for some common liquids. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure.
from www.slideserve.com
Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapor and saturation pressure for some common liquids. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid.
PPT Evaluation of VOC Definition Based on Vapor Pressure PowerPoint
Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapor and saturation pressure for some common liquids. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 23, pt 4 of 4 Example Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor and saturation pressure for some common liquids. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its. Vapor Definition Engineering.
From www.slideserve.com
PPT Evaluation of VOC Definition Based on Vapor Pressure PowerPoint Vapor Definition Engineering Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor and saturation pressure for some common liquids. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. At a given temperature,. Vapor Definition Engineering.
From www.slideserve.com
PPT Vaporization PowerPoint Presentation, free download ID3207099 Vapor Definition Engineering Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Vapor and saturation pressure for some common liquids. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. The. Vapor Definition Engineering.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapor and saturation pressure for some common liquids. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At. Vapor Definition Engineering.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 28, pt 1 of 7 Introduction Vapor Definition Engineering At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure is a fundamental concept in thermodynamics and physical. Vapor Definition Engineering.
From www.slideserve.com
PPT Chapter 1 Introduction to the Atmosphere PowerPoint Presentation Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Vapor and saturation pressure for some common. Vapor Definition Engineering.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Vapor and saturation pressure for some common. Vapor Definition Engineering.
From insulation.org
Understanding Thermal Systems Basic Cooling Systems Insulation Vapor Definition Engineering At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Vapor and saturation pressure. Vapor Definition Engineering.
From sciencenotes.org
Examples of Gases What Is a Gas? Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Vapor and saturation pressure for some common liquids.. Vapor Definition Engineering.
From www.slideserve.com
PPT Reaction Engineering Chemical Vapor Deposition CVD PowerPoint Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapor and saturation pressure for some common liquids. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour. Vapor Definition Engineering.
From www.slideserve.com
PPT Water Cycle Vocab PowerPoint Presentation, free download ID2405269 Vapor Definition Engineering Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. At a given temperature, the unique atmospheric pressure at. Vapor Definition Engineering.
From www.thermal-engineering.org
What is Cycle Refrigeration Cycle Definition Vapor Definition Engineering At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapor and saturation pressure for some common liquids. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor. Vapor Definition Engineering.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 Vapor Definition Engineering Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. At a given temperature, the unique atmospheric pressure at which a pure. Vapor Definition Engineering.
From in.pinterest.com
Vapor Pressure Easy Science Chemistry education, Learn biology Vapor Definition Engineering Vapor and saturation pressure for some common liquids. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. When. Vapor Definition Engineering.
From www.slideshare.net
Chapter 10 Lesson 4 Water Vapor and Humidity Vapor Definition Engineering Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Vapor and saturation pressure for some common liquids. At a given temperature,. Vapor Definition Engineering.
From www.slideserve.com
PPT Water Vapor in the Air PowerPoint Presentation, free download Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a given temperature, the unique atmospheric pressure at. Vapor Definition Engineering.
From www.exampleslab.com
15 Vaporization Examples Examples Lab Vapor Definition Engineering Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapor and saturation pressure for some common liquids. At a given temperature,. Vapor Definition Engineering.
From www.pipingengineer.org
Centrifugal Pump Terminology The Piping Engineering World Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapor and saturation pressure for some common liquids. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. At. Vapor Definition Engineering.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Vapor Definition Engineering Vapor and saturation pressure for some common liquids. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At. Vapor Definition Engineering.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Vapor Definition Engineering Vapor and saturation pressure for some common liquids. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. At. Vapor Definition Engineering.
From slideplayer.com
MATTER THE 3 STATES OF WATER ppt download Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapor and saturation pressure for some common liquids. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by. Vapor Definition Engineering.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapor and saturation pressure for some common liquids. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its. Vapor Definition Engineering.
From present5.com
Thermal physics Week 1 Vocabulary Understand Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapor and saturation pressure for some common liquids. The vapor pressure of a liquid is defined as the pressure exerted by. Vapor Definition Engineering.
From engineerexcel.com
Demystifying Saturated Vapor Properties and Applications EngineerExcel Vapor Definition Engineering At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapor and saturation pressure for some common liquids. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a.. Vapor Definition Engineering.
From www.thermal-engineering.org
What is Wet Steam Definition Vapor Definition Engineering Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. At a given temperature, the unique atmospheric. Vapor Definition Engineering.
From youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Vapor Definition Engineering Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapour pressure is a fundamental concept in thermodynamics and. Vapor Definition Engineering.
From www.slideserve.com
PPT Reaction Engineering Chemical Vapor Deposition CVD PowerPoint Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapour pressure is a fundamental concept in thermodynamics and physical. Vapor Definition Engineering.
From saylordotorg.github.io
Vapor Pressure Vapor Definition Engineering Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapor and saturation pressure for some common liquids. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. When. Vapor Definition Engineering.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson Vapor Definition Engineering At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapor and saturation pressure for some common liquids. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature. Vapor Definition Engineering.
From www.thoughtco.com
Vapor Definition Chemistry Glossary Vapor Definition Engineering Vapor and saturation pressure for some common liquids. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of. Vapor Definition Engineering.
From www.slideserve.com
PPT Vapor and it Pressure PowerPoint Presentation, free download ID Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor and saturation pressure for some common liquids. When vapor pressure equals. Vapor Definition Engineering.
From www.slideserve.com
PPT Evaluation of VOC Definition Based on Vapor Pressure PowerPoint Vapor Definition Engineering When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapour pressure is a fundamental concept in thermodynamics and physical chemistry, describing the pressure exerted by a. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Generally a substance's vapor pressure increases as. Vapor Definition Engineering.
From engineerexcel.com
Heat of Vaporization Explained EngineerExcel Vapor Definition Engineering Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Vapor and saturation pressure for some common liquids. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called its vapor pressure. Vapour pressure. Vapor Definition Engineering.
From gamesmartz.com
Water Vapor Definition Easy to Understand Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. Vapor and saturation pressure for some common liquids. At a given temperature, the unique atmospheric pressure at which a pure liquid boils is called. Vapor Definition Engineering.
From www.differencebetween.com
Difference Between Steam and Vapor Compare the Difference Between Vapor Definition Engineering The vapor pressure of a liquid is defined as the pressure exerted by the molecules that. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor and saturation pressure for some common liquids. When vapor pressure equals atmospheric pressure, the liquid starts to boil, leading to the formation of bubbles within the liquid. At. Vapor Definition Engineering.