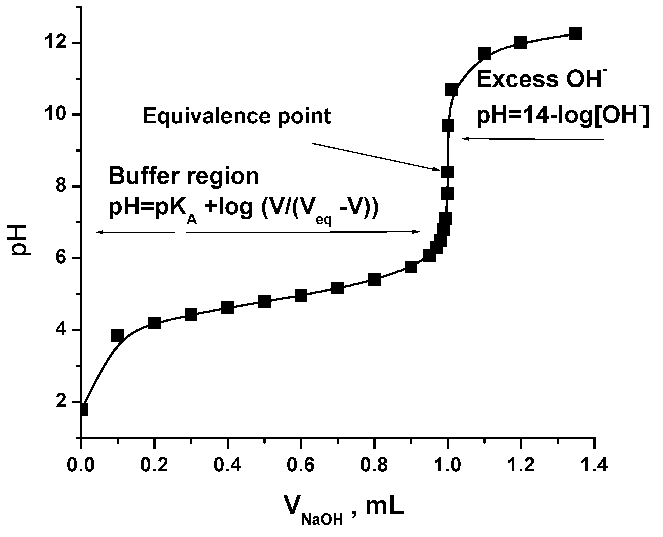
Endpoint Of A Titration Vs Equivalence Point . The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. The endpoint of a titration is the point where a color change occurs. The endpoint refers to the point in the experiment. The term neutral point is best avoided. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Difference between equivalence point and endpoint definition. Endpoint and equivalence point are two terms commonly used in titration experiments. The term equivalence point means that the solutions have been mixed in exactly the. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.
from goodttorials.blogspot.com
The endpoint refers to the point in the experiment. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. Endpoint and equivalence point are two terms commonly used in titration experiments. The term neutral point is best avoided. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Difference between equivalence point and endpoint definition. The term equivalence point means that the solutions have been mixed in exactly the. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. The endpoint of a titration is the point where a color change occurs. Equivalence point is the actual point where the chemical reaction in the titration mixture ends.
How To Find Equivalence Point From Titration Data
Endpoint Of A Titration Vs Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The term neutral point is best avoided. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Endpoint and equivalence point are two terms commonly used in titration experiments. Difference between equivalence point and endpoint definition. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. The endpoint of a titration is the point where a color change occurs. The term equivalence point means that the solutions have been mixed in exactly the. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Endpoint Of A Titration Vs Equivalence Point The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. The endpoint refers to the point in the experiment. The term neutral point is best avoided. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Endpoint Of A Titration Vs Equivalence Point.
From mungfali.com
Endpoint Titration Curve Endpoint Of A Titration Vs Equivalence Point The endpoint of a titration is the point where a color change occurs. The term equivalence point means that the solutions have been mixed in exactly the. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. Equivalence point is the actual point where the chemical reaction in the. Endpoint Of A Titration Vs Equivalence Point.
From animalia-life.club
Endpoint Titration Endpoint Of A Titration Vs Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. The term equivalence point means that the solutions have been mixed in exactly. Endpoint Of A Titration Vs Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Endpoint Of A Titration Vs Equivalence Point The endpoint of a titration is the point where a color change occurs. The term neutral point is best avoided. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. The term equivalence point means that the solutions have been mixed in exactly the.. Endpoint Of A Titration Vs Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Endpoint Of A Titration Vs Equivalence Point The endpoint of a titration is the point where a color change occurs. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Difference between equivalence point and endpoint definition. The endpoint refers to the point in the. Endpoint Of A Titration Vs Equivalence Point.
From edurev.in
End Point, Equivalence Point and Indicators Physical Chemistry PDF Endpoint Of A Titration Vs Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The term equivalence point means that the solutions have been mixed in exactly the. Equivalence point is the actual point where the chemical reaction in the titration mixture. Endpoint Of A Titration Vs Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Endpoint Of A Titration Vs Equivalence Point We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. The endpoint of a titration is the point where a color change occurs. The term neutral point is best. Endpoint Of A Titration Vs Equivalence Point.
From askanydifference.com
Endpoint vs Equivalence Point Difference and Comparison Endpoint Of A Titration Vs Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The endpoint of a titration is the point where a color change occurs. Endpoint. Endpoint Of A Titration Vs Equivalence Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Endpoint Of A Titration Vs Equivalence Point Difference between equivalence point and endpoint definition. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The endpoint of a titration is the point where a color change occurs. Equivalence point is the actual point where the. Endpoint Of A Titration Vs Equivalence Point.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Endpoint Of A Titration Vs Equivalence Point Difference between equivalence point and endpoint definition. The endpoint of a titration is the point where a color change occurs. The term equivalence point means that the solutions have been mixed in exactly the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). Endpoint Of A Titration Vs Equivalence Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Endpoint Of A Titration Vs Equivalence Point The endpoint refers to the point in the experiment. The endpoint of a titration is the point where a color change occurs. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the. Difference between equivalence point and endpoint definition. The main difference between an equivalence point and an endpoint. Endpoint Of A Titration Vs Equivalence Point.
From mungfali.com
Equivalence Points On Titration Graph Endpoint Of A Titration Vs Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Difference between equivalence point and endpoint definition. The term neutral point is best avoided. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. Endpoint Of A Titration Vs Equivalence Point.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Endpoint Of A Titration Vs Equivalence Point The endpoint refers to the point in the experiment. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The term neutral point is best avoided. The main difference between an equivalence point and an endpoint is that. Endpoint Of A Titration Vs Equivalence Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Endpoint Of A Titration Vs Equivalence Point Difference between equivalence point and endpoint definition. The endpoint refers to the point in the experiment. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. A titration is a volumetric technique in which. Endpoint Of A Titration Vs Equivalence Point.
From ar.inspiredpencil.com
Endpoint Titration Endpoint Of A Titration Vs Equivalence Point The term neutral point is best avoided. Difference between equivalence point and endpoint definition. The endpoint of a titration is the point where a color change occurs. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. A titration is a volumetric technique in which a solution of one. Endpoint Of A Titration Vs Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Endpoint Of A Titration Vs Equivalence Point The term neutral point is best avoided. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. The main difference between an equivalence point and an endpoint is that the former marks the end. Endpoint Of A Titration Vs Equivalence Point.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Endpoint Of A Titration Vs Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the. The endpoint refers to the point in the experiment. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. Equivalence point is the actual point where the chemical reaction in. Endpoint Of A Titration Vs Equivalence Point.
From www.pw.live
Difference Between Endpoint And Equivalence Point Endpoint Of A Titration Vs Equivalence Point Equivalence point is the actual point where the chemical reaction in the titration mixture ends. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. We can use this change in color to indicate the end point of a titration, provided that it occurs. Endpoint Of A Titration Vs Equivalence Point.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Endpoint Of A Titration Vs Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the. The endpoint refers to the point in the experiment. The term neutral point is best avoided. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. The main difference between. Endpoint Of A Titration Vs Equivalence Point.
From mavink.com
Titration Endpoint Vs Equivalence Point Endpoint Of A Titration Vs Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction. Endpoint Of A Titration Vs Equivalence Point.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Endpoint Of A Titration Vs Equivalence Point Difference between equivalence point and endpoint definition. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. The endpoint of a titration is the point where a color change. Endpoint Of A Titration Vs Equivalence Point.
From www.vrogue.co
Difference Between Endpoint And Equivalence Point1 Di vrogue.co Endpoint Of A Titration Vs Equivalence Point The endpoint of a titration is the point where a color change occurs. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Endpoint Of A Titration Vs Equivalence Point.
From mavink.com
Titration Endpoint Vs Equivalence Point Endpoint Of A Titration Vs Equivalence Point Equivalence point is the actual point where the chemical reaction in the titration mixture ends. The endpoint refers to the point in the experiment. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. Endpoint and equivalence point are two terms commonly used in. Endpoint Of A Titration Vs Equivalence Point.
From marielanewslee.blogspot.com
Explain the Difference Between End Point and Equivalence Point Endpoint Of A Titration Vs Equivalence Point Difference between equivalence point and endpoint definition. The term equivalence point means that the solutions have been mixed in exactly the. Endpoint and equivalence point are two terms commonly used in titration experiments. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. Equivalence. Endpoint Of A Titration Vs Equivalence Point.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Endpoint Of A Titration Vs Equivalence Point The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. A titration is. Endpoint Of A Titration Vs Equivalence Point.
From www.sciencemotive.com
Differentiate Between Endpoint and Equivalence Point ScienceMotive Endpoint Of A Titration Vs Equivalence Point The endpoint refers to the point in the experiment. Difference between equivalence point and endpoint definition. The term neutral point is best avoided. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. A titration is a volumetric technique in which a solution of. Endpoint Of A Titration Vs Equivalence Point.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free Endpoint Of A Titration Vs Equivalence Point Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Difference between equivalence point and endpoint definition. The endpoint refers to the point in the experiment. The term neutral point is best avoided. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near. Endpoint Of A Titration Vs Equivalence Point.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a Endpoint Of A Titration Vs Equivalence Point The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. The term neutral point is best avoided. The endpoint refers to the point in the experiment. The endpoint of a titration is the point where a color change occurs. The term equivalence point means. Endpoint Of A Titration Vs Equivalence Point.
From www.animalia-life.club
Endpoint Titration Endpoint Of A Titration Vs Equivalence Point The endpoint refers to the point in the experiment. The endpoint of a titration is the point where a color change occurs. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. We can use this change in. Endpoint Of A Titration Vs Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Endpoint Of A Titration Vs Equivalence Point We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. Difference between equivalence point and endpoint definition. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Endpoint Of A Titration Vs Equivalence Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Endpoint Of A Titration Vs Equivalence Point Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Difference between equivalence point and endpoint definition. We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. A titration is a volumetric technique in which a solution of one. Endpoint Of A Titration Vs Equivalence Point.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Endpoint Of A Titration Vs Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the. The endpoint of a titration is the point where a color change occurs. Difference between equivalence point and endpoint definition. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. The main difference between an equivalence point and an endpoint is. Endpoint Of A Titration Vs Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Endpoint Of A Titration Vs Equivalence Point We can use this change in color to indicate the end point of a titration, provided that it occurs at or near the titration’s equivalence point. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. Endpoint and equivalence point are two terms commonly. Endpoint Of A Titration Vs Equivalence Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Endpoint Of A Titration Vs Equivalence Point Difference between equivalence point and endpoint definition. Endpoint and equivalence point are two terms commonly used in titration experiments. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. A titration is a volumetric technique in which a solution of one reactant (the titrant). Endpoint Of A Titration Vs Equivalence Point.
From www.vrogue.co
Endpoint Vs Equivalence Point Understanding The Diffe vrogue.co Endpoint Of A Titration Vs Equivalence Point The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point. Difference between equivalence point and endpoint definition. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Endpoint Of A Titration Vs Equivalence Point.